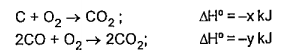A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- The values of DeltaH and DeltaS of a certain reaction are -400 kJ mol^...
Text Solution
|
- The enthalpy of vaporization of a certain liquid at its boiling point ...
Text Solution
|
- Given that The heat of formation of carbon monoxide will be
Text Solution
|
- The value of DeltaH for cooling 2 mole of an ideal monoatomic gas from...
Text Solution
|
- For a spontaneous process, the correct statement(s) is (are)
Text Solution
|
- Which of the following statements is correct for the spontaneous absor...
Text Solution
|
- For the reaction X2O4(l) to 2XO2(g), DeltaU = 2.1 kcal, DeltaS = 20 ...
Text Solution
|
- The ratio of heats liberated at 298K from the combustion of one kg of ...
Text Solution
|
- Conversion of oxygen into ozone is non-spontaneous at
Text Solution
|
- For an ideal binary liquid mixture
Text Solution
|
- The work done when one mole of an idea! gas is compressed form a volum...
Text Solution
|
- Assuming enthalpy of combustion of hydrogen at 273K is -286 kJ/mol and...
Text Solution
|
- A piston filled with 0.04mole of an ideal gas expa nds teversibly from...
Text Solution
|
- If DeltaG for a reaction is negative, you infer that the change is (1...
Text Solution
|
- In order to decompose 9g water 142.5 kJ heat is required. Hence, the e...
Text Solution
|
- The enthalpy of combustion of C6H6(l) is -3250 kJ. When 0.39 g of benz...
Text Solution
|
- The intensive property among these quantities is
Text Solution
|
- Hess's law states that
Text Solution
|
- A gas can expand from 100 mL to 250 mL. under a constant pressure of 2...
Text Solution
|
- For a Process to be spontaneous
Text Solution
|