A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- Standard enthalpy of vaporisation, Delta(vap)H^theta for water at 100^...
Text Solution
|
- Equal volumes of two monoatomic gases, A and B, at same temperature an...
Text Solution
|
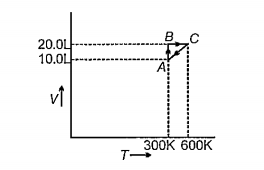
- This graph expresses the various steps of the system containing 1 mol ...
Text Solution
|
- Which of the following is correct ?
Text Solution
|
- On passing C ampere of current for time ‘t' sec through 1L of 2 (M) Cu...
Text Solution
|
- Entropy of the universe is
Text Solution
|
- The equilibrium constant of a reaction is 0.008 at 298 K. The standard...
Text Solution
|
- A gas undergoes a thermodynamical process but the volume of the gas do...
Text Solution
|
- The occurence of reaction is impossible if
Text Solution
|
- The bond dissociation energies of XY,X2 and Y2 (all diatomic molecules...
Text Solution
|
- Work done during isothermal expension of one mole of an ideal gas from...
Text Solution
|
- The amount of the heat released when 20 ml 0.5M NaOH is mixed with 100...
Text Solution
|
- A plot of In k against 1/T (abscissa) is expected to be a straight lin...
Text Solution
|
- Based on the first law of thermodynamics, which one of the following i...
Text Solution
|
- The value of enthalpy change (DeltaH) for the reaction C2H5OH(l) + 3O2...
Text Solution
|
- Consider the reaction, 4NO2(g) + O2(g) overset to 2N2O5,(g), DeltarH =...
Text Solution
|
- Molar heat capacity of aluminum is 25 JK^-1 mol^-1. The heat necessary...
Text Solution
|
- The entropy change involed in the isothermal reversible expansion of 2...
Text Solution
|
- In view of the signs of △ r G ∘ for the following reactions PbO...
Text Solution
|
- For the reversible reaction, A(s) + B(g) iff C(g) + D(g), DeltaG^@ = -...
Text Solution
|