A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- Enthalpy change for the reaction, 4H(g) overset to 2H2(g) is -869.5 kJ...
Text Solution
|
- If the enthalpy change for the transition of liquid water to steam is ...
Text Solution
|
- Based on the first law of thermodynamics, which one of the following i...
Text Solution
|
- The enthalpy of solution of sodium chloride is 4 kj mol^-1 and its ent...
Text Solution
|
- Four grams of graphite is burnt in a bomb calorimeter of heat capacity...
Text Solution
|
- Which reaction, with the following values of DeltaH, DeltaS at 400K is...
Text Solution
|
- The enthalpy of vaporisation of benzene is +35.3 kJ/mol at its boiling...
Text Solution
|
- For the reversible reaction, A(s) + B(g) iff C(g) + D(g), DeltaG^@ = -...
Text Solution
|
- A reaction is spontaneous at low temperature but non spontaneous at hi...
Text Solution
|
- At the sublimition temperture, for the process CO2(s) iff CO2(g) (1) ...
Text Solution
|
- Match the thermodynamic processes given under Column I with the expres...
Text Solution
|
- For the process H2O(i) to H2O(g) at T = 100^@C and 1 atmospere pressur...
Text Solution
|
- For complete combustion of ethanol, C2H5OH(l), + 3O2(g) to 2CO2(g) + ...
Text Solution
|
- The standard enthalpies of formation of CO2(g), H2O(l) and glucose (s)...
Text Solution
|
- A fixed mass m of a gas is subjected to transformation of states from ...
Text Solution
|
- A fixed mass m of a gas is subjected to transformation of states from ...
Text Solution
|
- For an ideal gas, consider only p-V work in going from an initial stat...
Text Solution
|
- Using the data provided calculate the multiple bond energy (KJ mol^(-)...
Text Solution
|
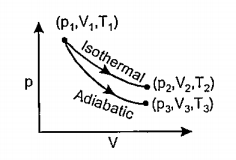
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- A gas expands from a volume of 1 m^3 to a volume of 2 m^3 against an e...
Text Solution
|