Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-P-BLOCK ELEMENTS -QUESTION BANK
- Silicon is second most abundant element occurring in earth crust. It i...
Text Solution
|
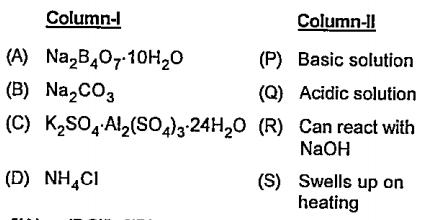
- Match Column-I with Column-II
Text Solution
|
- Match Column-I with Column-II
Text Solution
|
- Match Column-I with Column-II
Text Solution
|
- The number of sp^2 hybridized boron atoms in borax
Text Solution
|
- On combustion of 1 mole of glucose the number of moles of CO2 produced...
Text Solution
|
- The minimum number of moles of HCl required to completely react with 1...
Text Solution
|
- In sheet silicate how many oxygen atoms of [SiO4]^(4-) are shared ?
Text Solution
|
- Starting from boric acid, how would you prepare (i) meta and (ii) tetr...
Text Solution
|
- Boron tribromide is stronger acid than boron trifluoride. Why ?
Text Solution
|
- Can we prepare anhydrous AlCl3 by heating AlCl3. 6H2O ?
Text Solution
|
- Aluminium fluoride is ionic while AlCl3 is covalent. Why ?
Text Solution
|
- Explain the following "The ppi-ppi back bonding occurs in the halide...
Text Solution
|
- Compound (X) on reduction with LiAlH4 gives a hydride (Y) containing 2...
Text Solution
|
- Carbon tetrachloride is not affected but silicon tetrachloride is hydr...
Text Solution
|
- Why Sn (II) is a reducing agent whereas Pb (II) is not ?
Text Solution
|
- Graphite is a conductor but diamond is not a conductor. Explan.
Text Solution
|
- PbCl4 exist while PbBr4 and PbI4 do not exist.-Explain.
Text Solution
|
- Borax structure contains
Text Solution
|
- When orthoboric acid is heated strongly, it gives which of the followi...
Text Solution
|