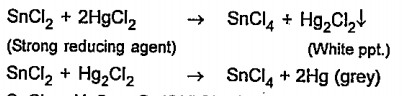Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-P-BLOCK ELEMENTS -QUESTION BANK
- In B2H6 how many hydrogen can be replaced by CH3 groups.
Text Solution
|
- Why is SiH4 more reactive than CH4 ?
Text Solution
|
- SnCl2 gives white precipitate with HgCl2 which turns grey later on, bu...
Text Solution
|
- A piece of Sn foil is added to SnCl2 solution for preserving it. Expla...
Text Solution
|
- An aqueous solution of a substance gives a white precipitate on treatm...
Text Solution
|
- AlCl3 fumes in moist air because of :
Text Solution
|
- The stability of +1 oxidation state among Al, Ga, In and TI increases ...
Text Solution
|
- Pyrosilicate ion is
Text Solution
|
- Name of the alloy of aluminium which is used in aeroplane is
Text Solution
|
- Aluminium oxide is not reduced by chemical reactions since
Text Solution
|
- Which of the following structure is similar to graphite ?
Text Solution
|
- Which of these is not a monomer for a high molecular mass silicone pol...
Text Solution
|
- The basic structural unit of silicates is
Text Solution
|
- Assertion PbI4 is a stable compound Reason iodide stabilizes higher ...
Text Solution
|
- Water gas is produced by
Text Solution
|
- Philosopher's wool on heating with BaO at 1100^@C produce
Text Solution
|
- The composition of duralumin is
Text Solution
|
- The molecular formula of cryolite is
Text Solution
|
- Sindoor is represented by
Text Solution
|
- Correct formula of aluminium nitride is
Text Solution
|