Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R G PUBLICATION-SOLUTION-EXERCISE
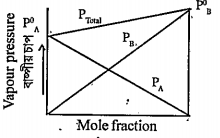
- A plot is obtained for two volatile liquids A & B as follows. From the...
Text Solution
|
- A plot is obtained for two volatile liquids A & B as follows. From the...
Text Solution
|
- A plot is obtained for two volatile liquids A & B as follows. From the...
Text Solution
|
- Solution of two volatile liquids A and B obey Roalt's law. At certain...
Text Solution
|
- The density of lake water is found to be 1.25 g mL^(-1) and contains 9...
Text Solution
|
- Explain when a non volatile solute is added to a liquid solvent, the v...
Text Solution
|
- Write three necessary conditoins for an ideal solution.,
Text Solution
|
- Calculate the mass of a non-volatile solute of molar mass 40 which sh...
Text Solution
|
- What is colligative property? Show that this property is proportional ...
Text Solution
|
- Define Ebulliscopic constant (Kb). Write its S.I. unit. Between water ...
Text Solution
|
- 2g of a non-electrolyte solute dissolved in 100g of benzene lowered th...
Text Solution
|
- Suppose you are required to determine the molecular mass of a protein ...
Text Solution
|
- Explain hypertonic and hypotonic solution?
Text Solution
|
- A 1.2% solution of NaCl is isotonic with 7.2% solution of glucose. Cal...
Text Solution
|
- A fresh carrot becomes dry in dry season . Why?
Text Solution
|
- What is reverse osmosis? Discuss one of its application?
Text Solution
|
- Show that for dimerisation of benzoic acid in benzene solution, degree...
Text Solution
|
- Calculate Van't Hoff factor 'i' for 0.001 molal NaCl solution.
Text Solution
|
- In a cold climate water gets forzen causing damage to the radiator of...
Text Solution
|
- Suggest the most important type of intermolecular interaction in the f...
Text Solution
|