Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R G PUBLICATION-CHEMICAL KINETICS-EXERCISE
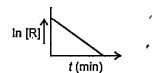
- For a chemical reaction variation in concentraction, ln[R] vs. time (...
Text Solution
|
- For a chemical reaction variation in concentraction, ln[R] vs. time (...
Text Solution
|
- For a chemical reaction variation in concentraction, ln[R] vs. time (...
Text Solution
|
- For the reaction 2N2O5(g) rarr 4NO2(g)+O2(g) the following results h...
Text Solution
|
- For the reaction 2N2O5(g) rarr 4NO2(g)+O2(g) the following results h...
Text Solution
|
- For the reaction 2N2O5(g) rarr 4NO2(g)+O2(g) the following results h...
Text Solution
|
- Show that for a first order reaction, the half life is independed of t...
Text Solution
|
- Identify the reaction order from each of the following rate constants....
Text Solution
|
- Identify the reaction order from each of the following rate constants....
Text Solution
|
- The conversion of molecule A to B follows second order kinetics. If co...
Text Solution
|
- Give the defination of collision frequency.
Text Solution
|
- For the reaction R rarr P the rate becomes 4 times faster when the con...
Text Solution
|
- Show that integrated rate law for the first order reaction R rarr P is...
Text Solution
|
- A first order reaction takes 40minutes for 20% decomposition. Calculat...
Text Solution
|
- A reaction is second order with respect to a reactant. How is the rate...
Text Solution
|
- Show that time required for completion 3/4th of a first order reaction...
Text Solution
|
- For a reaction 2A rarr 4B+C, the concentration of B is increased by 5....
Text Solution
|
- Show that slope of the plot of Ink against 1/T is -(Ea)/R. Give the gr...
Text Solution
|
- Starting from the intergrated rate law of a zeroth order reaction R ra...
Text Solution
|
- Starting from the intergrated rate law of a zeroth order reaction R ra...
Text Solution
|