Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R G PUBLICATION-CHEMICAL KINETICS-EXERCISE
- In a pseudo first order hydrolysis of ester in water the following res...
Text Solution
|
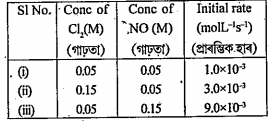
- The date below are for the reaction of NO and Cl2 to form NOCl at 295K...
Text Solution
|
- The date below are for the reaction of NO and Cl2 to form NOCl at 295K...
Text Solution
|
- The date below are for the reaction of NO and Cl2 to form NOCl at 295K...
Text Solution
|
- The date below are for the reaction of NO and Cl2 to form NOCl at 295K...
Text Solution
|
- During nuclear explosion one of the products is ^90Sr with half life ...
Text Solution
|
- Show that for a first order reaction the time required for 75% complet...
Text Solution
|
- The experimental data for decomposition of N2O5 in a gas phase at 318K...
Text Solution
|
- The experimental data for decomposition of N2O5 in a gas phase at 318K...
Text Solution
|
- The experimental data for decomposition of N2O5 in a gas phase at 318K...
Text Solution
|
- The decomposition of NH3 on plantinum surface is a zero order reaction...
Text Solution
|
- The half-life for radioactive decay of C-14 is 57830 year. An archaeol...
Text Solution
|
- A zero order reaction is 50% complete in 10 mins. What percentage woul...
Text Solution
|
- Discuss the effect of temperature on reaction rate.
Text Solution
|
- An endothermnic reaction A rarr B has an activation energy 15kJ//mol a...
Text Solution
|
- The rate constant for the decomposition of hydrocarbons is 2.418xx10^(...
Text Solution
|
- The decomposition of hydrocarbon follows the equation k = (4.5xx10^11 ...
Text Solution
|
- What are the functions of catalyst in a reaction?
Text Solution
|
- Discuss the collision theory of reaction rate.
Text Solution
|
- The time required for 10% completion of a first order reaction at 298 ...
Text Solution
|