Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R G PUBLICATION-CHEMICAL KINETICS-EXERCISE
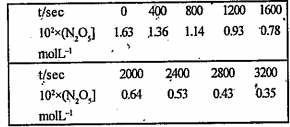
- The experimental data for decomposition of N2O5 in a gas phase at 318K...
Text Solution
|
- The experimental data for decomposition of N2O5 in a gas phase at 318K...
Text Solution
|
- The experimental data for decomposition of N2O5 in a gas phase at 318K...
Text Solution
|
- The decomposition of NH3 on plantinum surface is a zero order reaction...
Text Solution
|
- The half-life for radioactive decay of C-14 is 57830 year. An archaeol...
Text Solution
|
- A zero order reaction is 50% complete in 10 mins. What percentage woul...
Text Solution
|
- Discuss the effect of temperature on reaction rate.
Text Solution
|
- An endothermnic reaction A rarr B has an activation energy 15kJ//mol a...
Text Solution
|
- The rate constant for the decomposition of hydrocarbons is 2.418xx10^(...
Text Solution
|
- The decomposition of hydrocarbon follows the equation k = (4.5xx10^11 ...
Text Solution
|
- What are the functions of catalyst in a reaction?
Text Solution
|
- Discuss the collision theory of reaction rate.
Text Solution
|
- The time required for 10% completion of a first order reaction at 298 ...
Text Solution
|
- The activation energy of a certain uncatalysed reaction at 300K is 76k...
Text Solution
|
- Rate constant K for a first order reaction has been found to be 2.54xx...
Text Solution
|
- A first order gas phase reaction A2B2(g) rarr 2A(g)+2B(g) at the tempe...
Text Solution
|
- In a first order reaction, the concentration of the reactant is reduce...
Text Solution
|
- The half life for the first order reaction is 5xx10^4 sec. What percen...
Text Solution
|
- In Arrhenius equation What does the term e^(-E//RT) signify?
Text Solution
|
- In Arrhenius equation Can activation energy E for a reaction be zero...
Text Solution
|