Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R G PUBLICATION-THE d-AND f-BLOCK ELEMENTS-EXERCISE
- Write four factors which affect the relative stability of various oxid...
Text Solution
|
- Why E^@ values for Mn and Ni are negative?
Text Solution
|
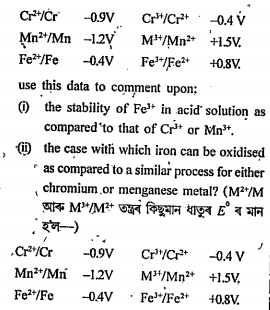
- For M^(2+)/M and M^(3+)/M^(2+) systems the E^@ values for some metals ...
Text Solution
|
- Why is the highest oxidation state of a metal exhibited in its oxide o...
Text Solution
|
- Explain the following facts:transition elements form coloured compound...
Text Solution
|
- Explain the following facts: Transition elements and their compounds s...
Text Solution
|
- Explain the following facts: transition elements form complex compound...
Text Solution
|
- Explain the following facts: Transition metals form alloy
Text Solution
|
- Explain the following facts: Transition elements form interstitial com...
Text Solution
|
- How K2Cr2O7 is prepared? Write the necessary reactions involved.
Text Solution
|
- What happens when potassium dichromate reacts with H2S and KI in acidi...
Text Solution
|
- Indicate the steps in the preparation of KMnO4.
Text Solution
|
- Write the cause of lenthanide contraction.
Text Solution
|
- Actinoid contraction is greater from element to element than lanthanoi...
Text Solution
|
- What is meant by disproporation'. Give two examples of disproporationa...
Text Solution
|
- Compare the chemistry of actinoids with that of the lanthanoids with s...
Text Solution
|
- Write three applications of d-block elements.
Text Solution
|
- Explain the following: Cu(I) ion is not known is aquous solution
Text Solution
|
- How would you account for the following? The enthalpies of atomization...
Text Solution
|
- Explain the following: Co (II) is stable in aquous solution but in the...
Text Solution
|