Similar Questions
Explore conceptually related problems
Recommended Questions
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
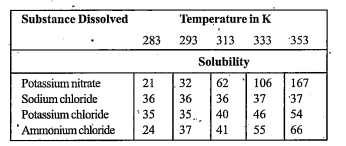
- Pargya tested the solubility of four different substances at different...
Text Solution
|
- प्रज्ञा ने तीन अलग-अलग पदार्थों की घुलनशीलताओं को अलग-अलग तापमान पर जा...
Text Solution
|
- Pragya tested the solubility of three different temperatures and colle...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|