A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-MOCK TEST 5-EXERCISE
- Hyperconjugation is more pronounced in
Text Solution
|
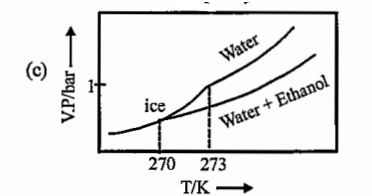
- Ice and water are placed in a closed container at a pressure of 1 atm ...
Text Solution
|
- The value of the 'spin only magnetic moment for one of the following c...
Text Solution
|
- Here[Y] is a
Text Solution
|
- Gradual addition of KI solution to Bi(NO3)(3) solution initially produ...
Text Solution
|
- If the average life time of an excited state of hydrogen is of the ord...
Text Solution
|
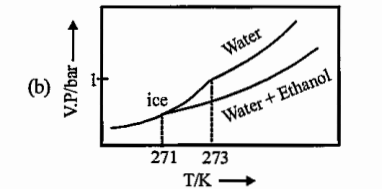
- Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethan...
Text Solution
|
- The major products obtained from the following sequence of reactions ...
Text Solution
|
- The root mean square velocity of an ideal gas to constant pressure var...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
- To an acidic solution of an anion, a few drops of Kmno(4) solution are...
Text Solution
|
- Select pair of compounds in which both have different hybridization bu...
Text Solution
|
- Which one of the following compounds would have the highest heat of hy...
Text Solution
|
- The rate constant, the activation energy and the Arrhenius parameter o...
Text Solution
|
- For a 'C'M concentarted solution of a weak electrolyte A(x)B(y)alpha(d...
Text Solution
|
- 3g of actived chacoal was added to 50mL of acetic acid solution (0.06N...
Text Solution
|
- The standard state Gibbs free energies of formation of ) C(graphite an...
Text Solution
|
- Li forms a body-centred cubic lattice. If the edge of the cube is 3.5 ...
Text Solution
|
- In the following reaction sequence in aqueous solution, the species X,...
Text Solution
|
- The gas phase decomposition of dimethyl ether follows first order kine...
Text Solution
|