A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-MOCK TEST 8-EXERCISE
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- The enthalpy change of the reaction C3H8(g)+H2(g) rarr C2H6(g)+CH4(g)...
Text Solution
|
- The pH of blood stream is maintained by a proper balance of H(2)CO(3) ...
Text Solution
|
- The number of beta-particles emitted during the change "aX^c rarr "dY^...
Text Solution
|
- An element is placed in second group and third group ofthe periodic ta...
Text Solution
|
- A freshly obtained of SnO(2) is peptized by little of KOH to give a so...
Text Solution
|
- To prepare 3-ethylpentan-3-ol, the reactants needed are
Text Solution
|
- The follwing diagram indicates the energy levels of a certain atom whe...
Text Solution
|
- KCl crystallises in the same type of lattice as does NaCl Given that r...
Text Solution
|
- When phosphine is bubbled through a solution of nitrate is precipitat...
Text Solution
|
- The correct order of acidity for the following compounds is
Text Solution
|
- The equalitative sketches I, II and III given below show the variation...
Text Solution
|
- Which of the following statements is not true?
Text Solution
|
- The major product of the following reaction sequence is
Text Solution
|
- Experimentally it was found that a metal oxide in formula M(0.98)O. Me...
Text Solution
|
- Product (B) is:
Text Solution
|
- Two grams of benzoic acid (C(6)H(5)COOH) dissolved in 25.0 g of benzen...
Text Solution
|
- Among the complex ions, [Co(NH2–CH2–CH2–NH2–)2Cl2^(])+, [CrCl2(C2O4)2]...
Text Solution
|
- An organic compound ‘A’ on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- N(2) + 3 H(2) rarr 2NH(3) Molecular weight of NH(3) and N(2) are x(1...
Text Solution
|
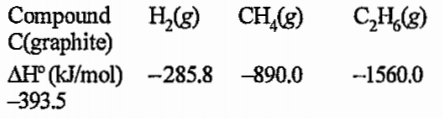 (The standard heat of formation of `C_3H_8(g)` is
-103.8kJ/mol)
(The standard heat of formation of `C_3H_8(g)` is
-103.8kJ/mol)