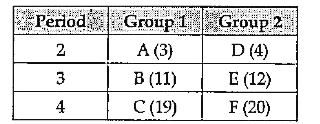Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-THE PERIODIC TABLE -TOPIC 2 Studying Individual Groups (5 Marks Questions)
- (a)Name the first three alkaline earth metals. (b) Write their react...
Text Solution
|
- The table given below shows the position of element U, V, X, Y and Z ...
Text Solution
|
- The table given below shows the position of element U, V, X, Y and Z ...
Text Solution
|
- In the table given below, six elements are shown with their atomic nu...
Text Solution
|
- In the table given below, six elements are shown with their atomic nu...
Text Solution
|
- In the table given below, six elements are shown with their atomic nu...
Text Solution
|
- In the table given below, six elements are shown with their atomic nu...
Text Solution
|
- In the table given below, six elements are shown with their atomic nu...
Text Solution
|
- Complete the following table :
Text Solution
|
- An element with atomic number 5 belongs to III A group of the modern p...
Text Solution
|
- An element with atomic number 5 belongs to III A group of the modern p...
Text Solution
|
- An element with atomic number 5 belongs to III A group of the modern p...
Text Solution
|
- An element with atomic number 5 belongs to III A group of the modern p...
Text Solution
|
- An element with atomic number 5 belongs to III A group of the modern p...
Text Solution
|
- An element with atomic number 5 belongs to III A group of the modern p...
Text Solution
|
- From the standpoint of atomic structure, what determines which element...
Text Solution
|
- How does valency and valence electrons vary on moving from left to rig...
Text Solution
|
- Name the following: An alkaline earth metal in period 3 of the per...
Text Solution
|
- The elements of the third period of the Periodic Table are given below...
Text Solution
|
- Metalloid in 3rd period.
Text Solution
|