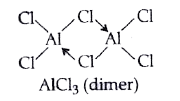Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 4-Questions
- How will you convert ethane to methane?
Text Solution
|
- A hydrocarbon decolourised bromine water. On ozonolysis it gives 3-met...
Text Solution
|
- Discuss the structure of aluminium chloride.
Text Solution
|
- Why does chromium have configuration of type 3d^(5) 4s^(1) instead of ...
Text Solution
|
- How many electrons possess anticlockwise spin in an atom of oxygen?
Text Solution
|
- At 27^@C, a cylinder of 20 L capacity contains three gases He, O2 " an...
Text Solution
|
- 750 mL of nitrogen are collected over water at 25^@C and 740 mm pressu...
Text Solution
|
- Out of 4s and 3d, which subshell is filled first and why?
Text Solution
|
- In potassium, the 19th electron enter into 4s subshells instead of 3d ...
Text Solution
|
- Calculate the entropy change (DeltaS) for the following reaction at 25...
Text Solution
|
- Define heat of formation.How is it useful in the calculation of the he...
Text Solution
|
- Calculate the calorific value of sugar if its heat of combustion is 56...
Text Solution
|
- Define air pollution. What are the main pollutants ?
Text Solution
|
- Distinguish between primary and secondary air pollutants.
Text Solution
|
- Indicate the sigma and pi bonds in the following molecules : C(6)H(...
Text Solution
|
- Why is +l-effect of t-butyl group greater than that of isopropyl group...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|