Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 4-Questions
- Define air pollution. What are the main pollutants ?
Text Solution
|
- Distinguish between primary and secondary air pollutants.
Text Solution
|
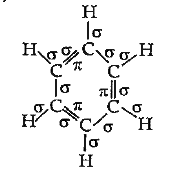
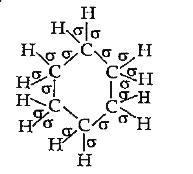
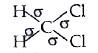
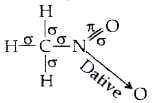
- Indicate the sigma and pi bonds in the following molecules : C(6)H(...
Text Solution
|
- Why is +l-effect of t-butyl group greater than that of isopropyl group...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|
- Justify that the following reactions are redox reactions : 2K(s)+F(2...
Text Solution
|
- Justify that the following reactions are redox reactions : 4NH(3)(g)...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- How do you account for the following observations ? Though alkaline ...
Text Solution
|
- How do you account for the following observations ? When concentrate...
Text Solution
|
- Explain why Alkanes with odd number of carbon atoms possess lower boil...
Text Solution
|
- Explain why Teflon is used in making non-stick cooking utensils.
Text Solution
|
- How will you convert propene to propane
Text Solution
|
- How will you bring out the following conversions ? Ethyne to but-2...
Text Solution
|