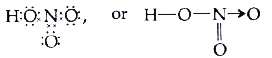Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 4-Questions
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Give the electron dot structure of the following compounds : SO2 ,...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|
- Justify that the following reactions are redox reactions : 2K(s)+F(2...
Text Solution
|
- Justify that the following reactions are redox reactions : 4NH(3)(g)...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- Calculate the oxidation number of the underlined atoms in the followin...
Text Solution
|
- How do you account for the following observations ? Though alkaline ...
Text Solution
|
- How do you account for the following observations ? When concentrate...
Text Solution
|
- Explain why Alkanes with odd number of carbon atoms possess lower boil...
Text Solution
|
- Explain why Teflon is used in making non-stick cooking utensils.
Text Solution
|
- How will you convert propene to propane
Text Solution
|
- How will you bring out the following conversions ? Ethyne to but-2...
Text Solution
|
- How would you convert the following compounds into benzene? (i) Ethy...
Text Solution
|
- Write IUPAC names of the products obtained by the ozonolysis of the fo...
Text Solution
|
- Write IUPAC names of the products obtained by the ozonolysis of the fo...
Text Solution
|
- How will you convert benzene into (a) p-nitrobromobenzene (b) m-nitrob...
Text Solution
|