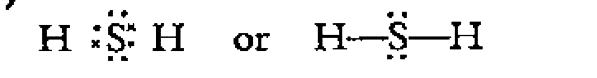Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER-Question
- What is tautomerism ? Give two examples.
Text Solution
|
- Write the keto and enolic forms of acetone.
Text Solution
|
- Draw the Lewis structures of the following species : H2 S
Text Solution
|
- Draw the Lewis structures for the following molecules and ion: CO(3...
Text Solution
|
- Draw the Lewis structures for the following molecules and ion: HCOOH
Text Solution
|
- Calculate the oxidation number of the underlined element in the follow...
Text Solution
|
- Calculate the oxidation number of the underlined element in the follow...
Text Solution
|
- Calculate the oxidation number of the underlined element in the follow...
Text Solution
|
- Calculate the oxidation number of the underlined element in the follow...
Text Solution
|
- Justify giving reactions that among halogens, fluorine is the best oxi...
Text Solution
|
- Refer to the periodic table given in your book and now answer the foll...
Text Solution
|
- Refer to the periodic table given in your book and now answer the foll...
Text Solution
|
- In Ostwald’s process for the manufacture of nitric acid, the first ste...
Text Solution
|
- What is Wurtz reaction ? Explain with examples. What are its limitatio...
Text Solution
|
- What happens when acetylene is treated with ozone
Text Solution
|
- What happens when iodoform is heated with silver powder
Text Solution
|
- The alkyl halide C4 H9 Br (A) reacts with alcoholic KOH and gives an a...
Text Solution
|
- One mole of nitrogen is mixed with three moles of hydrogen in a 4 litr...
Text Solution
|
- The solubility product of PbCl(2) at 298K is 1.7 xx 10^(-5). Calculate...
Text Solution
|
- The species: H(2)O, HCO(3)^(-), HSO(4)^(-) and NH(3) can act both as B...
Text Solution
|