Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RC MUKHERJEE-CHEMICAL KINETICS-OBJECTIVE PROBLEMS
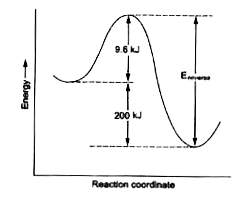
- The activation energy for the reaction , O(3) (g) + NO(g) to NO(2)...
Text Solution
|
- If the volume of a closed vessel in which the equilibrium 2SO(2)+O(2)h...
Text Solution
|
- The rate of the elementary reaction, 2NO+O(2)rarr 2NO(2), when the v...
Text Solution
|
- Rate of which reaction increase with temperature:
Text Solution
|
- The specific rate constant of a first order reaction depends on the
Text Solution
|
- If the rate constant k of a reaction is 1.6 times 10^–3(mol//l)(min^–...
Text Solution
|
- If for any raction , th rate constant is equal to the rate of the reac...
Text Solution
|
- Which of the following procedures will lead to a change in the rate co...
Text Solution
|
- If a reaction with t(1//2)=69.3 seconds , has a rate constant value o...
Text Solution
|
- The specfic reaction rate constant for a first order reaction is 1xx10...
Text Solution
|
- k for a zero order reaction is 2xx10^(-2) mol . L^(-1)s^(-1) . If the ...
Text Solution
|
- A first order reaction is carried out with an initial concentration of...
Text Solution
|
- Which of the following curves represents a third order reaction ?
Text Solution
|
- Which of the following curves represents a zero order reaction ? [(a-x...
Text Solution
|
- 75 % of a first order reaction was completed in 32 min . When was 50 %...
Text Solution
|
- If doubting the initial concentrations of a reactant doubles t(1/2) of...
Text Solution
|
- For a given reaction the logarithm of the concentration of the reactan...
Text Solution
|
- The concept of t(1/2) is useful for the reactions of
Text Solution
|
- The half - life for a given reaction was halved as the initial concent...
Text Solution
|
- The rate constant fro a second order reaction is 8 xx 10^(-5) M^(-1) "...
Text Solution
|
- The rate for a first -order reaction is 8 xx10^(-5) M^(-1)min^(-1). Ho...
Text Solution
|