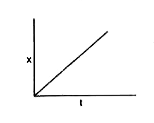
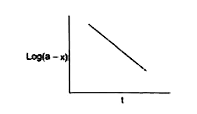
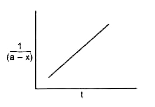
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RC MUKHERJEE-CHEMICAL KINETICS-OBJECTIVE PROBLEMS
- A first order reaction is carried out with an initial concentration of...
Text Solution
|
- Which of the following curves represents a third order reaction ?
Text Solution
|
- Which of the following curves represents a zero order reaction ? [(a-x...
Text Solution
|
- 75 % of a first order reaction was completed in 32 min . When was 50 %...
Text Solution
|
- If doubting the initial concentrations of a reactant doubles t(1/2) of...
Text Solution
|
- For a given reaction the logarithm of the concentration of the reactan...
Text Solution
|
- The concept of t(1/2) is useful for the reactions of
Text Solution
|
- The half - life for a given reaction was halved as the initial concent...
Text Solution
|
- The rate constant fro a second order reaction is 8 xx 10^(-5) M^(-1) "...
Text Solution
|
- The rate for a first -order reaction is 8 xx10^(-5) M^(-1)min^(-1). Ho...
Text Solution
|
- What fraction of a reactant remains after 40 min if t(1/2) is 20 min a...
Text Solution
|
- For a given reaction, the half-life period was found to be directly pr...
Text Solution
|
- From different sets of data of t(1//2) at diifferent initial concentra...
Text Solution
|
- A catalyst increases the rate of a chemical reaction by
Text Solution
|
- The energy of activation of a forward reaction is 50 kcal . The energy...
Text Solution
|
- The rate constant , the activation energy and the Arhenius parameter o...
Text Solution
|
- Which of the following statements is wrong about reactions ?
Text Solution
|
- The temperature coefficient of a reaction is 2 . The rate of this reac...
Text Solution
|
- The rate constant of a reaction , A to Product , with initial reactant...
Text Solution
|
- The rate constant of a reaction , A to Product ,with initial reactant...
Text Solution
|