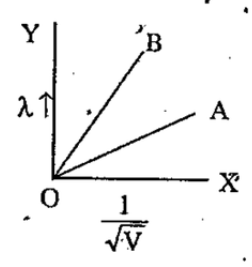Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
UNITED BOOK HOUSE-MODEL PAPER SET-05-EXERCISE
- State Biot Savart’s law.
Text Solution
|
- A ray of lilght is incident at a small angle theta on a rectangular gl...
Text Solution
|
- Write down the expression of angle of deviation by thin prism. A ray o...
Text Solution
|
- X-rays of wavelength lambda fall on photosensitive-surface emitting el...
Text Solution
|
- The two lines A and B shown in the graph plot the de Broglie wavelengt...
Text Solution
|
- What kind of diode is used as a voltage regulator? .draw the v-i char...
Text Solution
|
- How is the p-n junction, used as a half wave rectifier and draw the in...
Text Solution
|
- Write Kirchhoff’s 2nd law.
Text Solution
|
- For the network of conductors forming an electrical circuit as shown ...
Text Solution
|
- What is the nature of temperature coefficient of resistance of a semic...
Text Solution
|
- Group of N cells whose e.m.f. varies directly with the internal resist...
Text Solution
|
- Show that if n identical conductors are joined in series, the combined...
Text Solution
|
- It is said that the induced current has no direction of its own. DO yo...
Text Solution
|
- A closed circular coils of average radius 10 xx 10^-2 m is placed no...
Text Solution
|
- Show that in the C R circuit, the phase angle is tan^-1(1/(omegaCR))
Text Solution
|
- In an L-C-R circuit with all components connected in series,the e.m.f....
Text Solution
|
- In an L-C-R circuit with all components connected in series,the e.m.f....
Text Solution
|
- In an L-C-R circuit with all components connected in series,the e.m.f....
Text Solution
|
- In Young’s double slit experiment the distance between two slits is 0....
Text Solution
|
- In Young’s double slit experiment the distance between two slits is 0....
Text Solution
|