Similar Questions
Explore conceptually related problems
Recommended Questions
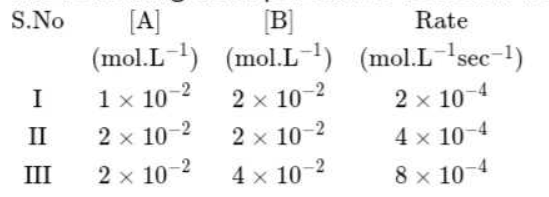
- The following data pertain to reaction between A and B Which ...
Text Solution
|
- For the reaction A + B rarr C + D , doubling the concentration of both...
Text Solution
|
- The following data pertain to reaction between A and B {:(,"S.No",[A],...
Text Solution
|
- In a reaction , A + B to C , the rate expression is R = K[A] [B]^(2...
Text Solution
|
- For the reaction A + B rarrC + D , doubling the concentration of both ...
Text Solution
|
- For the reaction A+2BrarrC , the reaction -rate is doubled if the conc...
Text Solution
|
- For the reaction A + B rarrC + D , doubling the concentration of both ...
Text Solution
|
- In a reaction A + B to Products (i) If the initial concentration of...
Text Solution
|
- For the reaction A + B rarr C + D, doubling the concentration of both ...
Text Solution
|