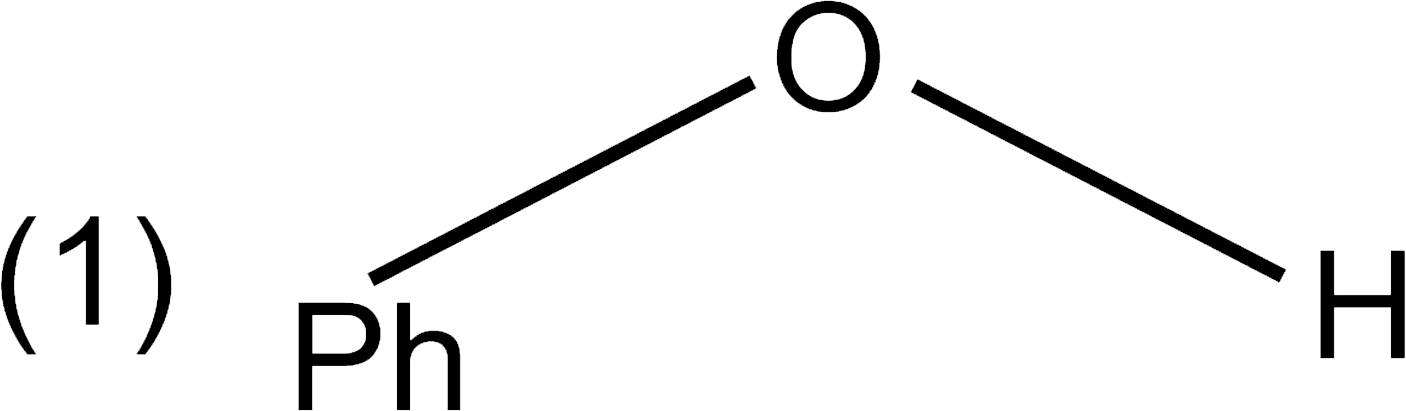
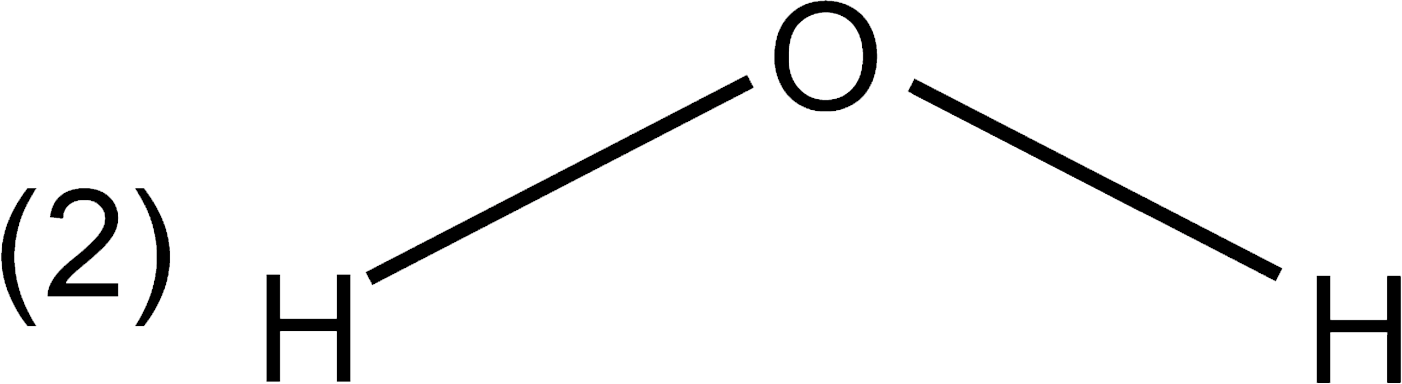
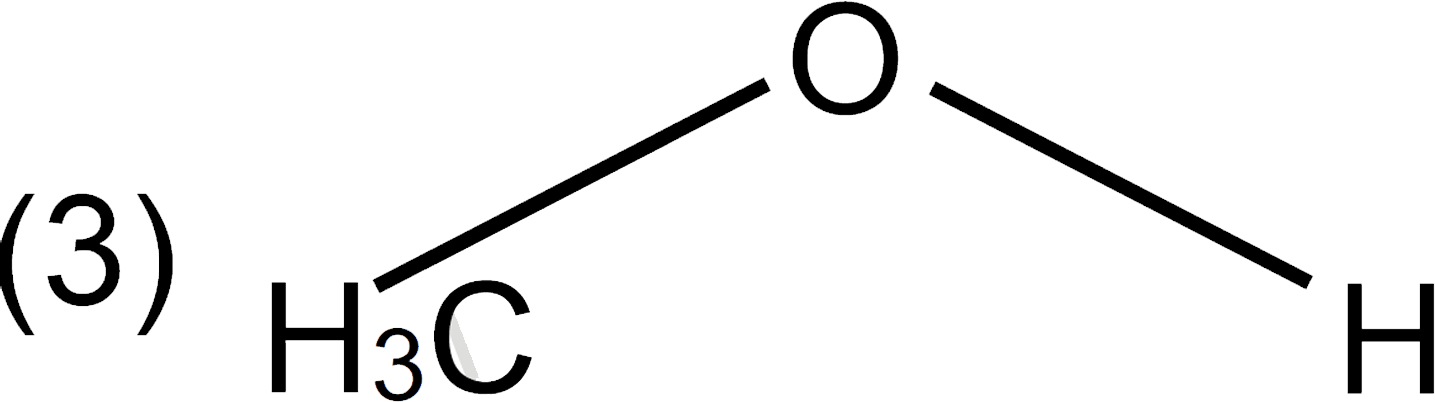
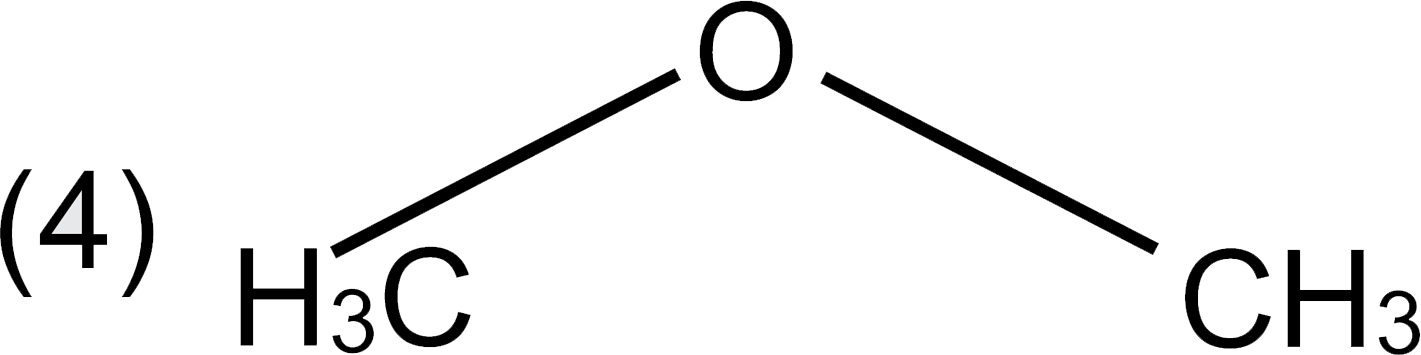
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compound that is most difficult protonate is
Text Solution
|
- The compound that is most difficult protonate is
Text Solution
|
- In which of the following compounds is hydroxylic proton the most acid...
Text Solution
|
- In which of the following compound is hydroxylic proton the most acidi...
Text Solution
|
- The most acidic proton and the strongent nucleophilic nitrogen in the ...
Text Solution
|
- Among the following, the compound having the most acidic proton is:
Text Solution
|
- फीनॉल का प्रोटोनीकरण कठिन है जबकि एथेनॉल का प्रोटोनीकरण आसानी से किया ...
Text Solution
|
- In which of the following compounds is hydroxylic proton the most acid...
Text Solution
|
- वह यौगिक जिसको प्रोटोनीकृत करना सर्वाधिक कठिन है, है
Text Solution
|