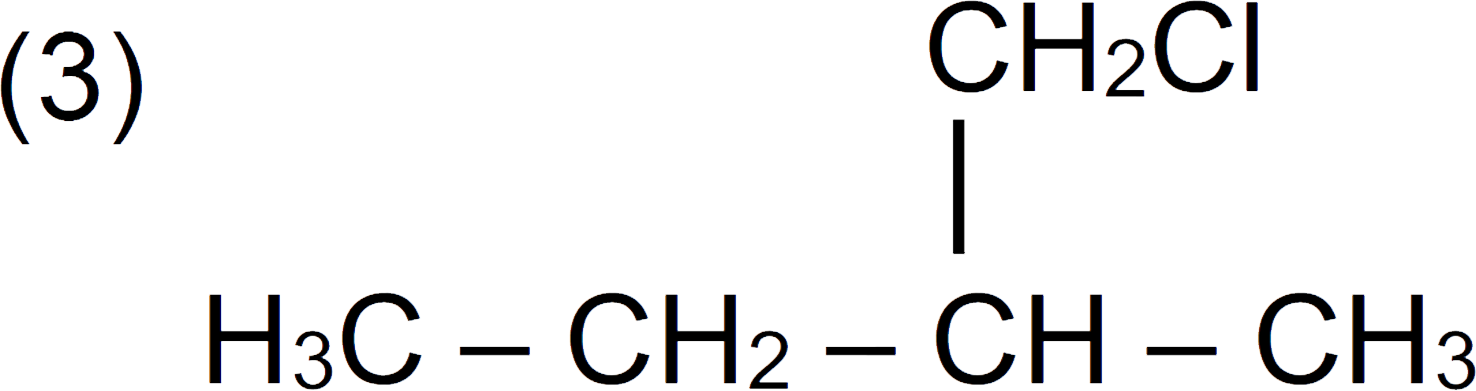A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An alkene "A" on reaction with O3 and Zn gives propanone and acetaldeh...
Text Solution
|
- An alkene "A" on reaction with O3 and Zn gives propanone and ethanol i...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with Markowni...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with Markowni...
Text Solution
|
- An alkene 'A' oz ozonolysis gives a mixture of ethanoal and pentan-3-o...
Text Solution
|
- Alkenes on treating with O3 and then the product on reduction may give
Text Solution
|
- An alkene ''A'' on reaction with O(3) and Zn-H(2)O gives propanone and...
Text Solution
|
- An alkene “A” on reaction with O3 and Zn - H2O gives propanone and eth...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with the Mark...
Text Solution
|