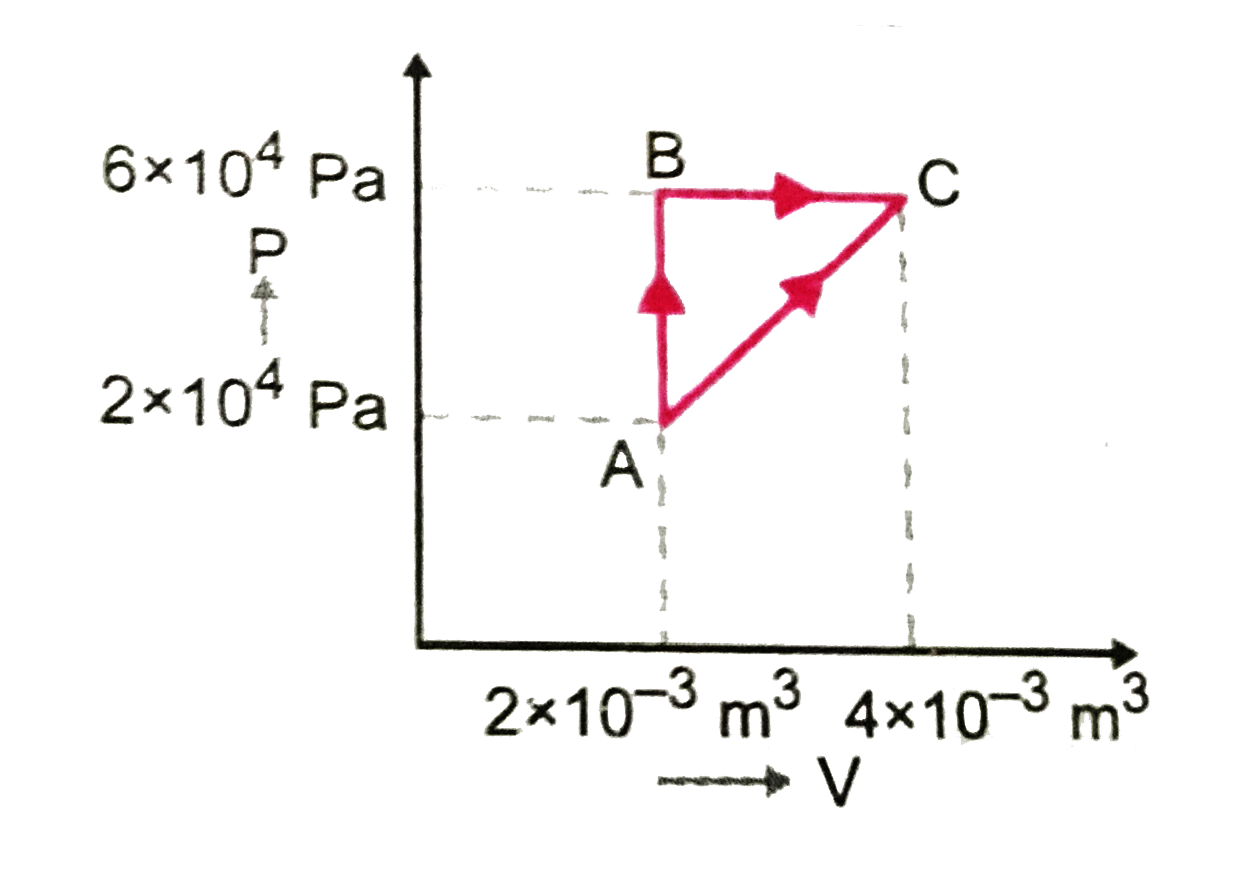
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In (figure). shows two path that may be taken by a gas to go from a st...
Text Solution
|
- In (figure). shows two path that may be taken by a gas to go from a st...
Text Solution
|
- When a system is taken from state A and B along the path ACB, 80J of h...
Text Solution
|
- In a process, 701 J of heat is absorbed by a system and 394 J of work ...
Text Solution
|
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|
- In a process 701 J of heat is absorbed by a system and 394 J of work i...
Text Solution
|
- In a process, 701 J of heat is absorbed by a system and 394 J of work ...
Text Solution
|
- Figure below shows two paths that may be taken by a gas to go from a s...
Text Solution
|
- Figure below shows two paths that may be taken by a gas to go from sta...
Text Solution
|