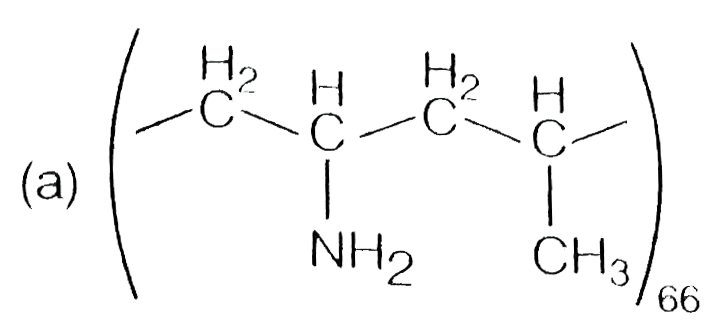
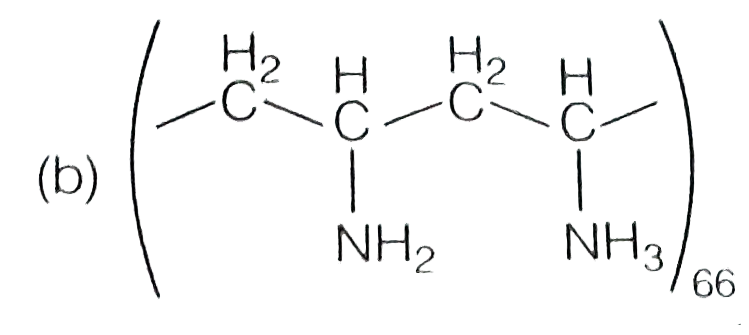
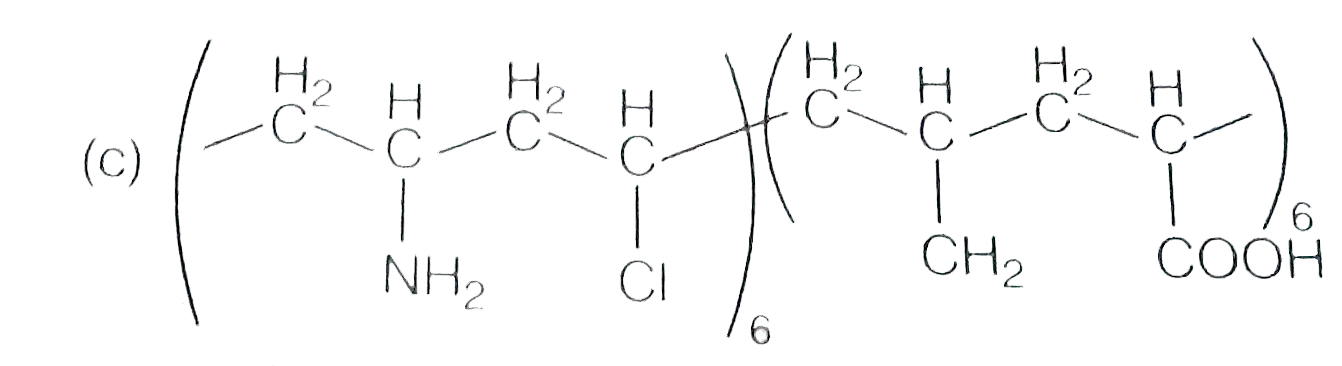
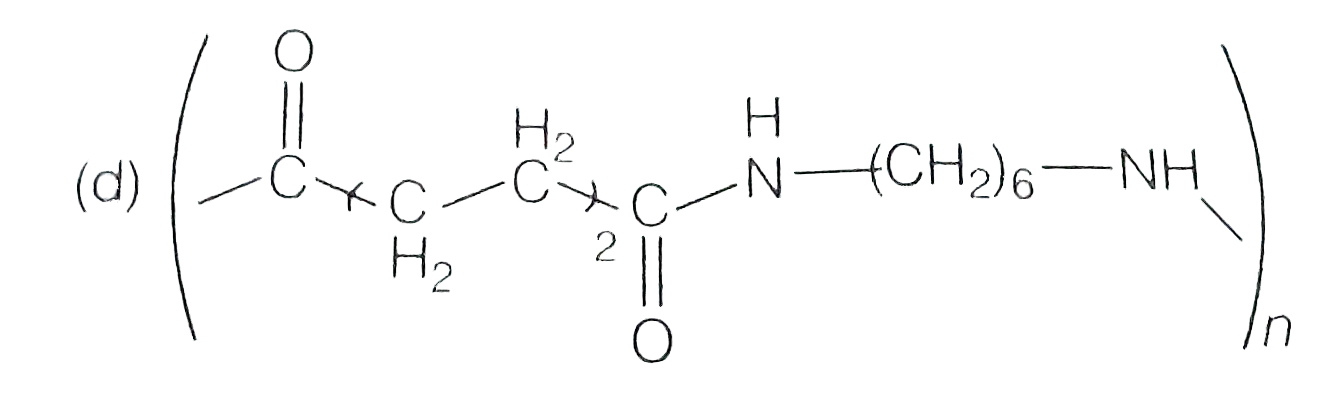
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following represent nylon 6,6 polymer?
Text Solution
|
- Which one of the following represent nylon 6,6 polymer?
Text Solution
|
- Which one of the following is not a polyamide polymers: (a) Nylon-6,...
Text Solution
|
- Which one of the following structures represents nylon-6,6 polymer?
Text Solution
|
- Which one of the following structures represents nylon-6,6 polymer?
Text Solution
|
- Which one of the following structures represents nylon 6,6 polymer ?
Text Solution
|
- निम्न में से कौन सी संरचना नायलॉन-6,6 बहुलक को प्रदर्शित करती है
Text Solution
|
- Which one of the following structures represents nylon 6,6 polymer?
Text Solution
|
- Which one of the following structures represents nylon-6,6 polymer ?
Text Solution
|