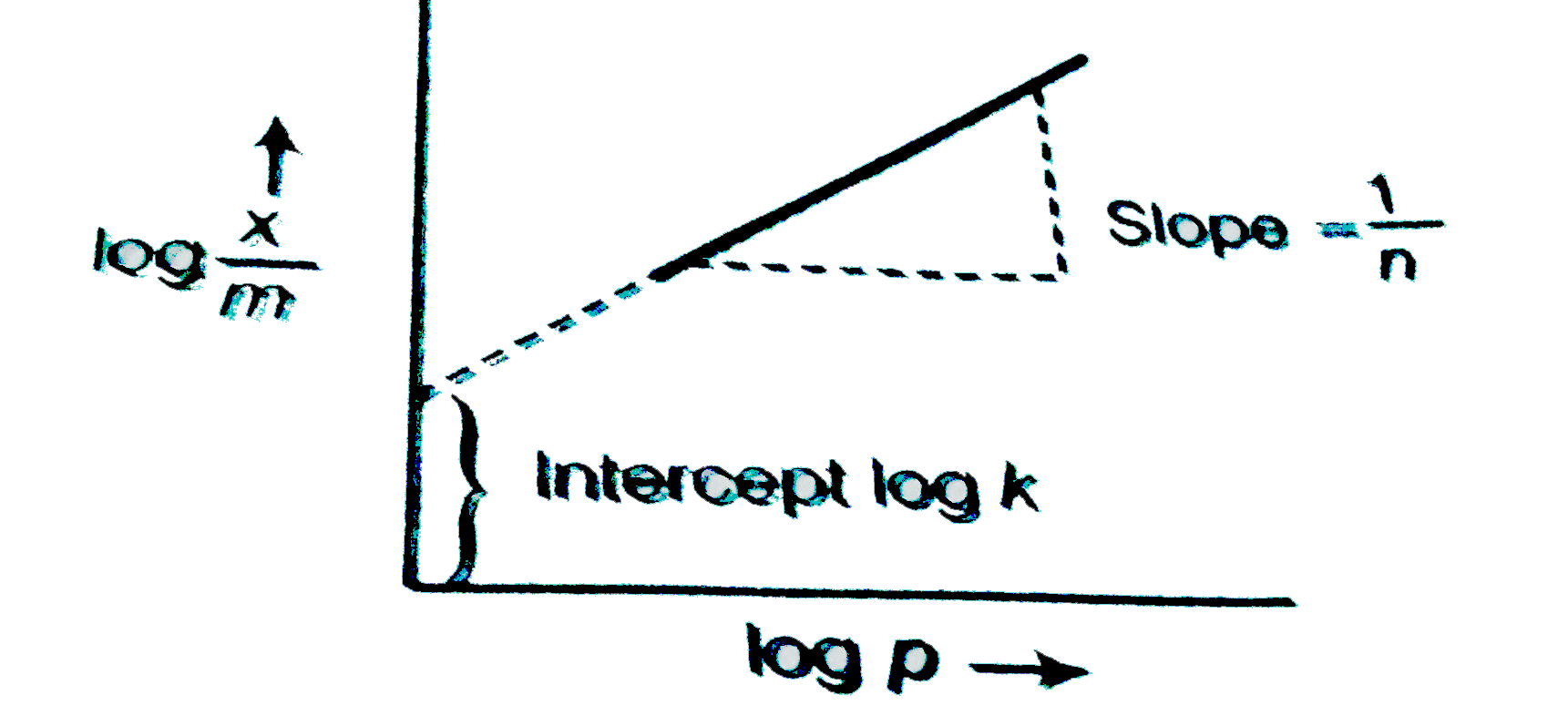
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A plot of log (x/m) against log P for the adsorption of a gas on a sol...
Text Solution
|
- A plt of log X/m vs. log P for the adsorption of a gasd on a solid giv...
Text Solution
|
- For adsorption of gas on solid suface. The plots of log x//m versus lo...
Text Solution
|
- A plot of log (x/m) against log P for the adsorption of a gas on a slo...
Text Solution
|
- A plot of x/m versus log p for the adsorption of a gas on a solid give...
Text Solution
|
- A plot of log (x/m) versus log p for the adsorption of a gas on the su...
Text Solution
|
- Adsorption of a gas on a surface follows Freundlich adsorption isother...
Text Solution
|
- A plot of log x/m versus log p for the adsorption of a gas on a solid ...
Text Solution
|
- For the adsorption of a gas on a solid, the plot of log(x/m) versus lo...
Text Solution
|