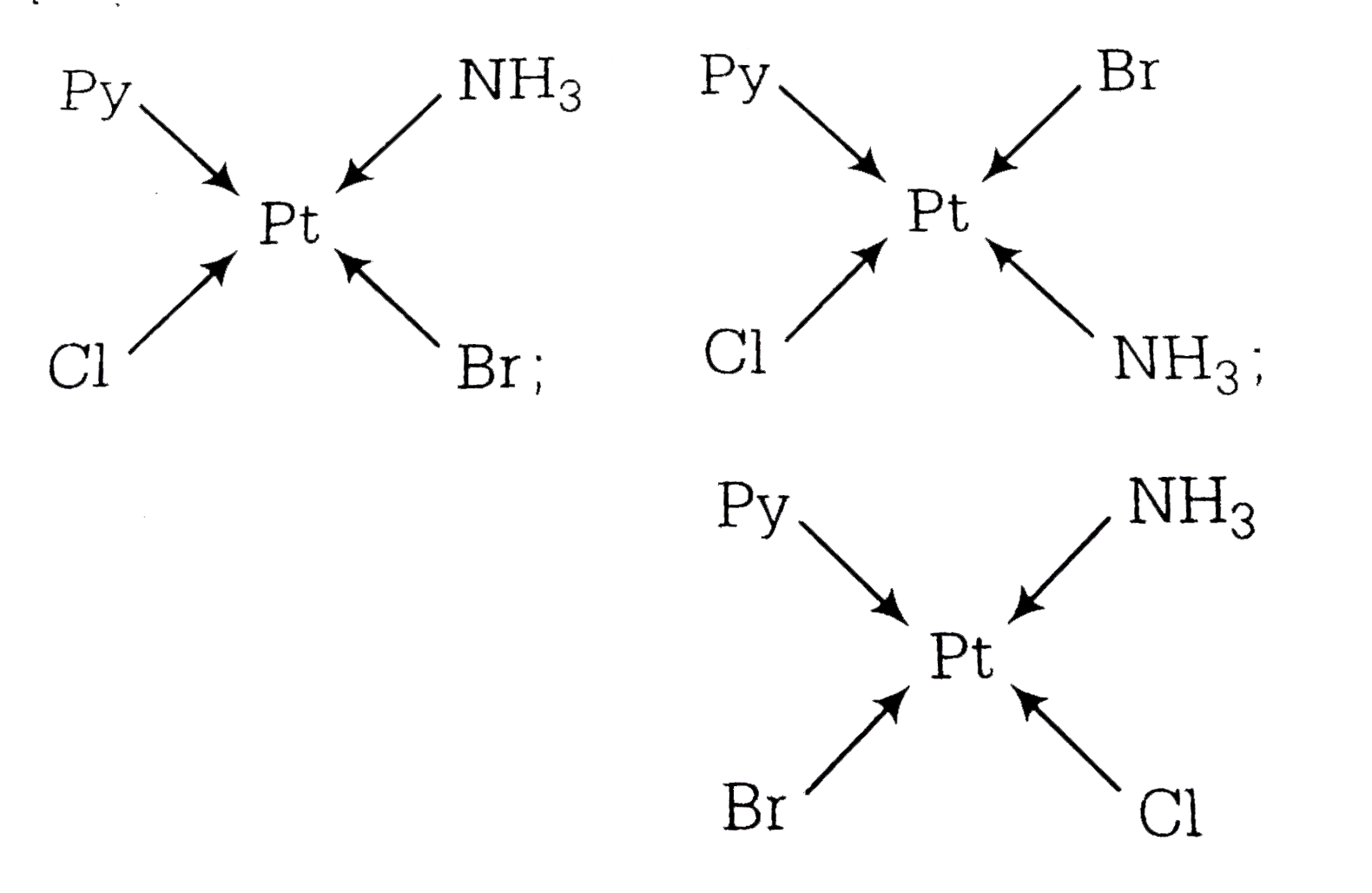
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The complex, [Pt(py)(NH3)BrCl] will have how many geometrical isomers?
Text Solution
|
- Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how man...
Text Solution
|
- The complex, [Pt(py)(NH3)BrCl] will have how many geometrical isomers?
Text Solution
|
- How many geometrical isomers and stereoisomers are possible for [Pt(NO...
Text Solution
|
- How many geometrical isomers are possible [Pt(py)(NH3) (Br)(Cl)]?
Text Solution
|
- The complex, [Pt(py)(NH(3))BrCl] will have how many geometical isomers...
Text Solution
|
- The complex, [Pt(Py)(NH(3))BrCl] will have how many geometrical isomer...
Text Solution
|
- [Pt(py)(NH3)BrCl] -এর জ্যামিতিক সমাবয়বের সংখ্যা
Text Solution
|
- Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how man...
Text Solution
|