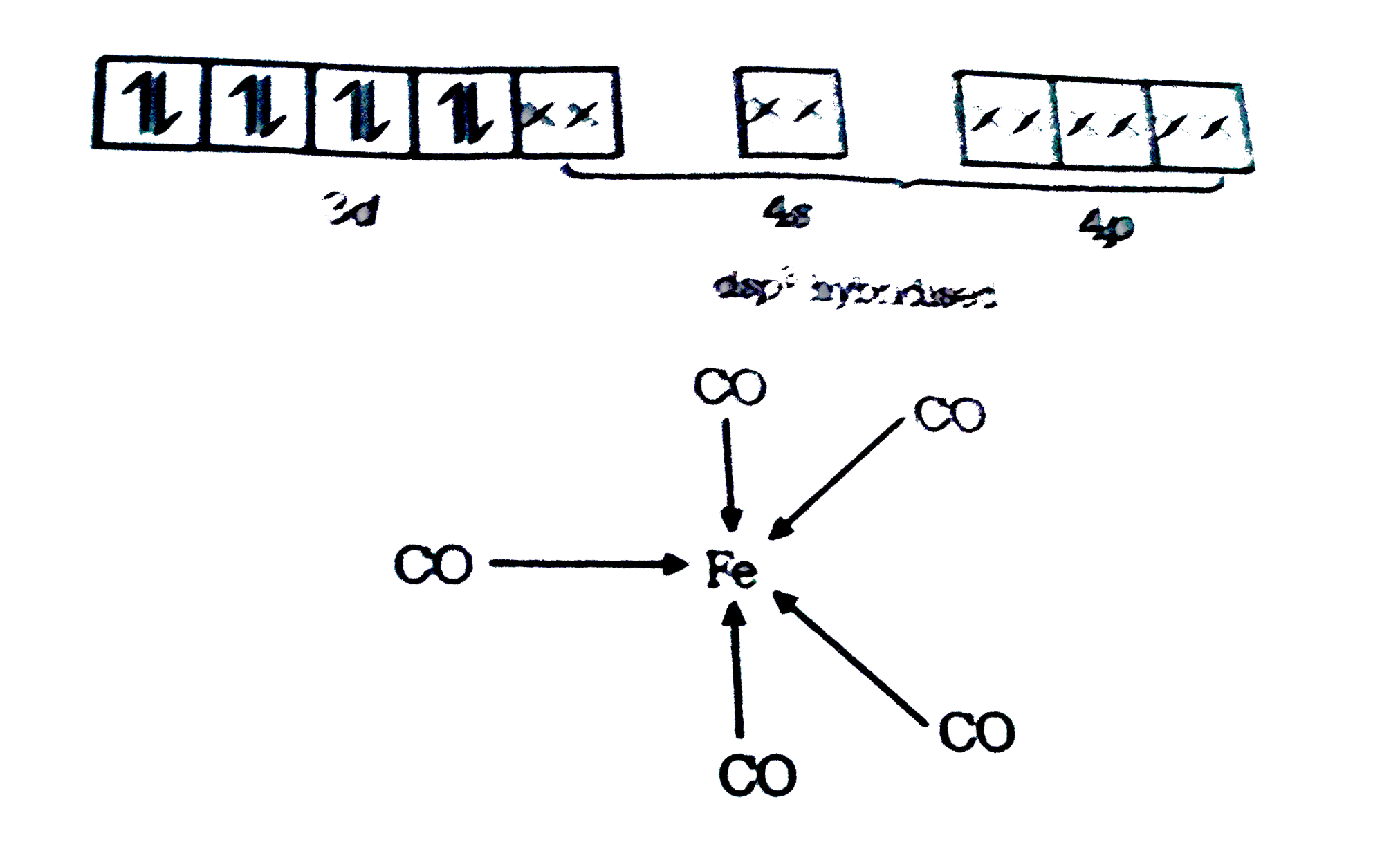A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The correct structure of Fe(CO)(5) is (Z = 26 for Fe)
Text Solution
|
- The correct structure of Fe(CO)(5) is (Z= 26 for Fe)
Text Solution
|
- The correct structure of Fe (CO)(5) is ?
Text Solution
|
- Correct configuration of Fe^"+3" [26] is
Text Solution
|
- Fe(CO)(5) की सही संरचना है:
Text Solution
|
- Fe ^(3 +) ( परमाणु क्रमांक Fe = 26 ) का सही विन्यास है -
Text Solution
|
- The number of d-electrons retained in Fe^(2+) (At. no. of Fe = 26) ion...
Text Solution
|
- The correct structure of Fe(CO)(5) is
Text Solution
|
- निम्न की संरचना लिखिए- Fe(CO)(5)
Text Solution
|