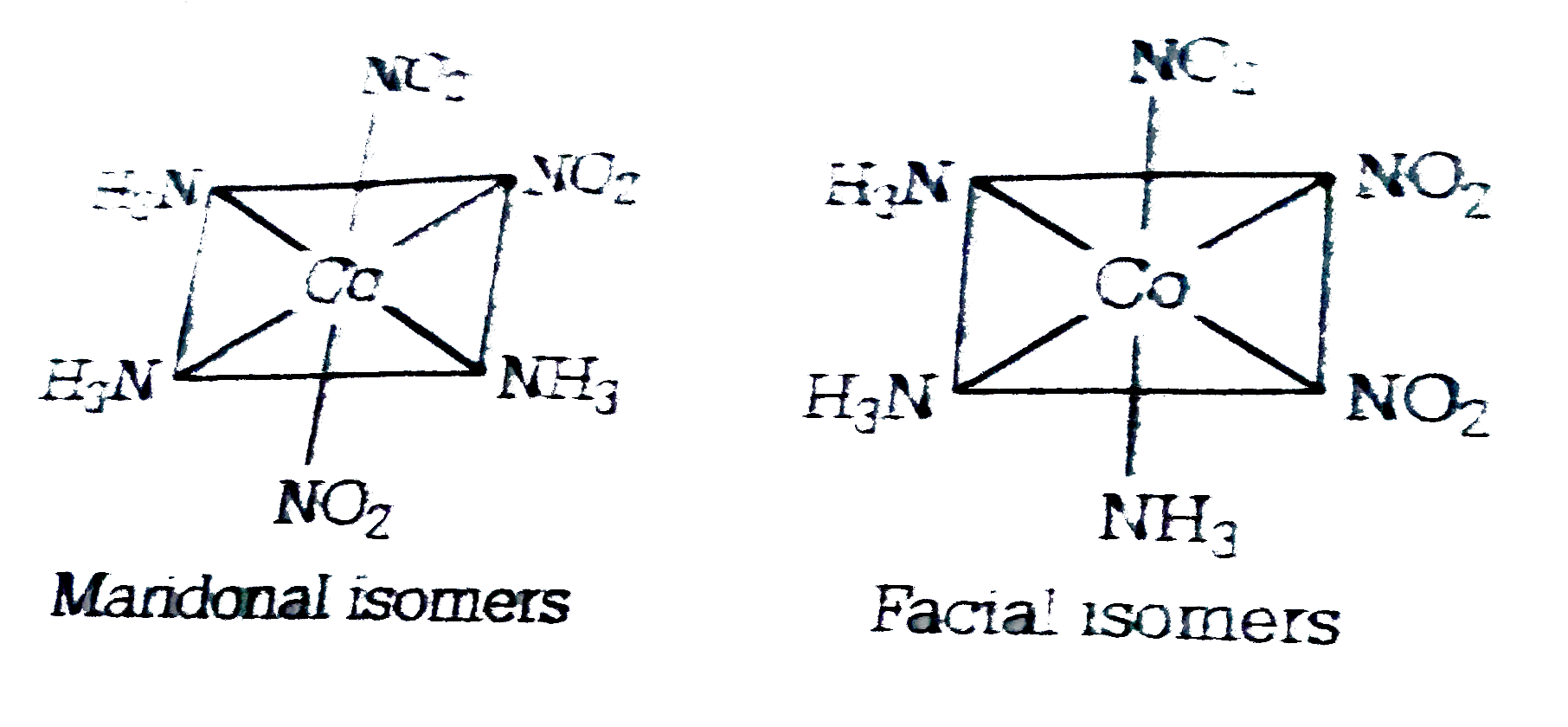A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of geometrical isomers of the complex [Co(NO(2))(3) (NH(3))...
Text Solution
|
- The number of geometrical isomers of the complex [Co(NO(2))(3) (NH(3))...
Text Solution
|
- Total number of geometrical isomers for the complex [Rh Cl (CO) (PPh(3...
Text Solution
|
- The number of geometrical isomers of [Co(NH(3))(3)(NO(3))(3)] is
Text Solution
|
- The total number of isomer shown by [Co(NH(3))(4)(NO(2))(2)](NO(3)) co...
Text Solution
|
- Choose the correct code regarding, possible number of geometrical isom...
Text Solution
|
- Total number of geometrical isomers for the complex [RhCl(CO)(PPh(3))(...
Text Solution
|
- The number of geometrical isomers of [Co(NH(3))(3)(NO(3))(3)] are :
Text Solution
|
- The number of geometrical isomers of [Co(NH(3))(3)(NO(3))(3)] are :
Text Solution
|