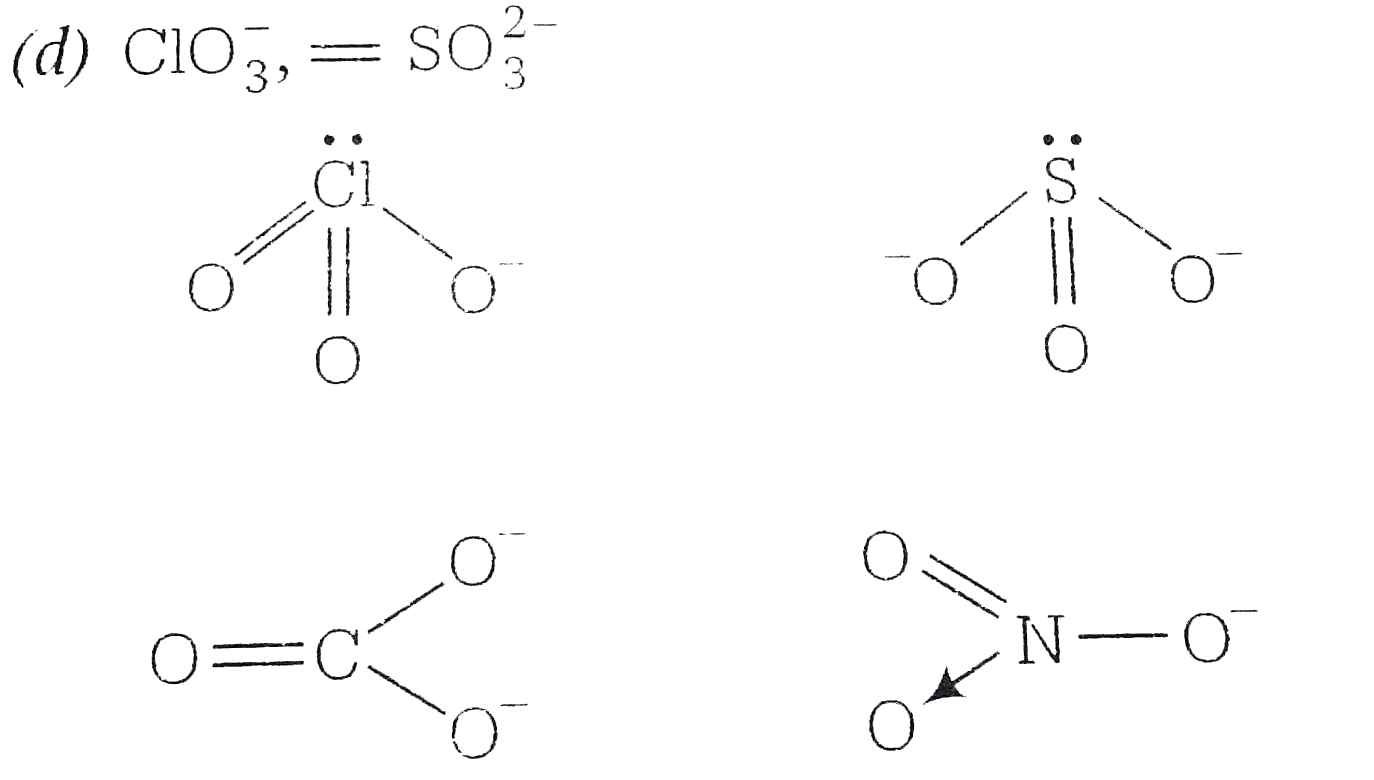
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and also isostr...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following pairs of ions is isoelectronic as well as isost...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|