A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
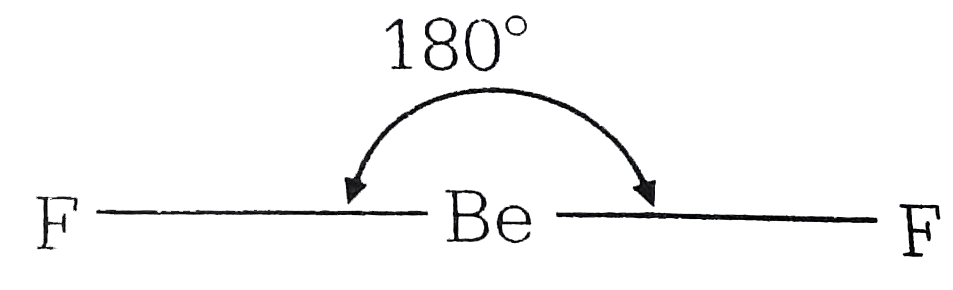
- H2O is dipolar, whereas BeF2 is not. It is because:-
Text Solution
|
- H2O is dipolar, whereas BeF2 is not. It is because
Text Solution
|
- BeF2 is soluble in water whereas the fluorides of other alkaline earth...
Text Solution
|
- H2O has net dipole moment while BeF2 has zero dipole moment because
Text Solution
|
- The bond angle in H2O is nearly 105^@ whereas bond angle in H2 S is ne...
Text Solution
|
- H2O has net dipole moment while BeF2 has zero dipole moment because
Text Solution
|
- H(2)O is dipolar whereas BeF(2) is not. It is because
Text Solution
|
- H2O is polar , whereas BeF2 is not . It is because .
Text Solution
|
- HF , H2O , BeF2 तथा NF3 अणुओं के द्विध्रुव आघुर्णों का घटता हुआ क्रम ...
Text Solution
|