A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
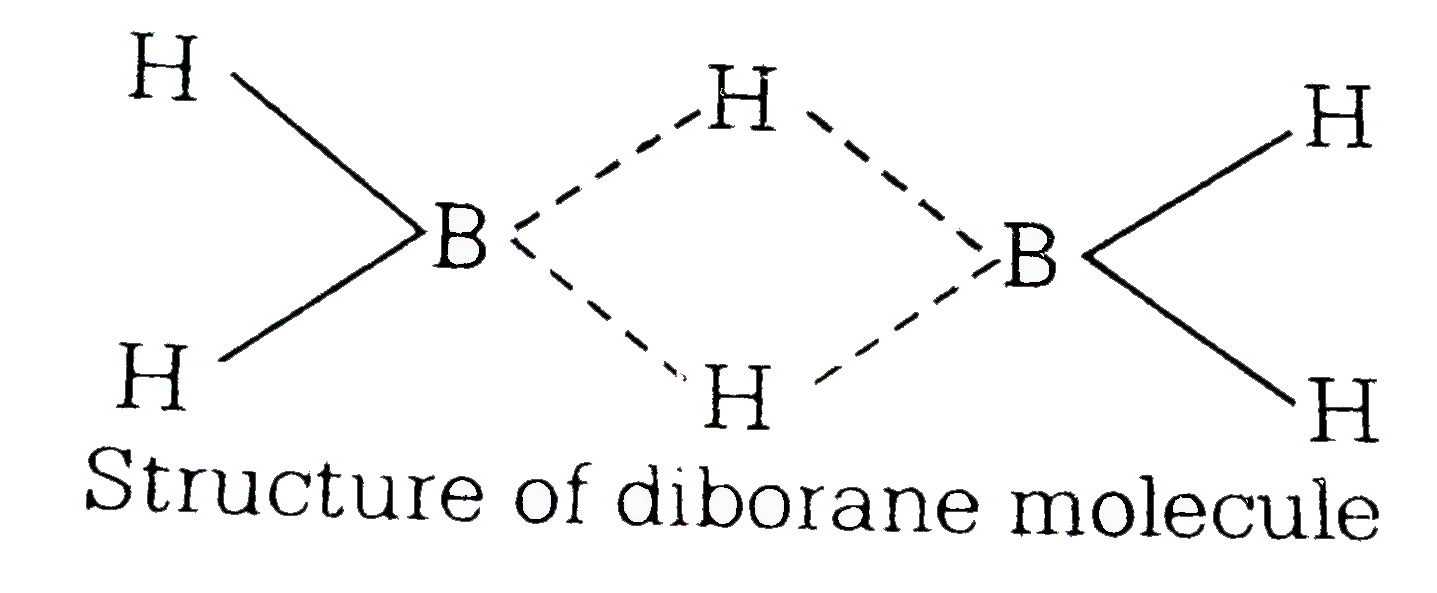
- The type of hybridization of boron in bibroane is
Text Solution
|
- The types of hybridisation of boron in diborane is
Text Solution
|
- The hybridization states of boron and nitrogen in borazole respectivel...
Text Solution
|
- The type of hybridization of boron in diborane is
Text Solution
|
- BF(3) में बोरॉन परमाणु की संकरण अवस्था क्या है?
Text Solution
|
- डाइबोरेन में बोरॉन का संकरण कौन-सा होता है?
Text Solution
|
- The type of hybridisation of boron in diborane is -
Text Solution
|
- What is the type of hybridization exhibited by Boron in Boron trifluor...
Text Solution
|
- సంకరీకరణం ఆధారంగా బోరాన్ ట్రై ఫ్లోరైడ్ అణువు ఏర్పడుటను వివరించండి.
Text Solution
|