A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
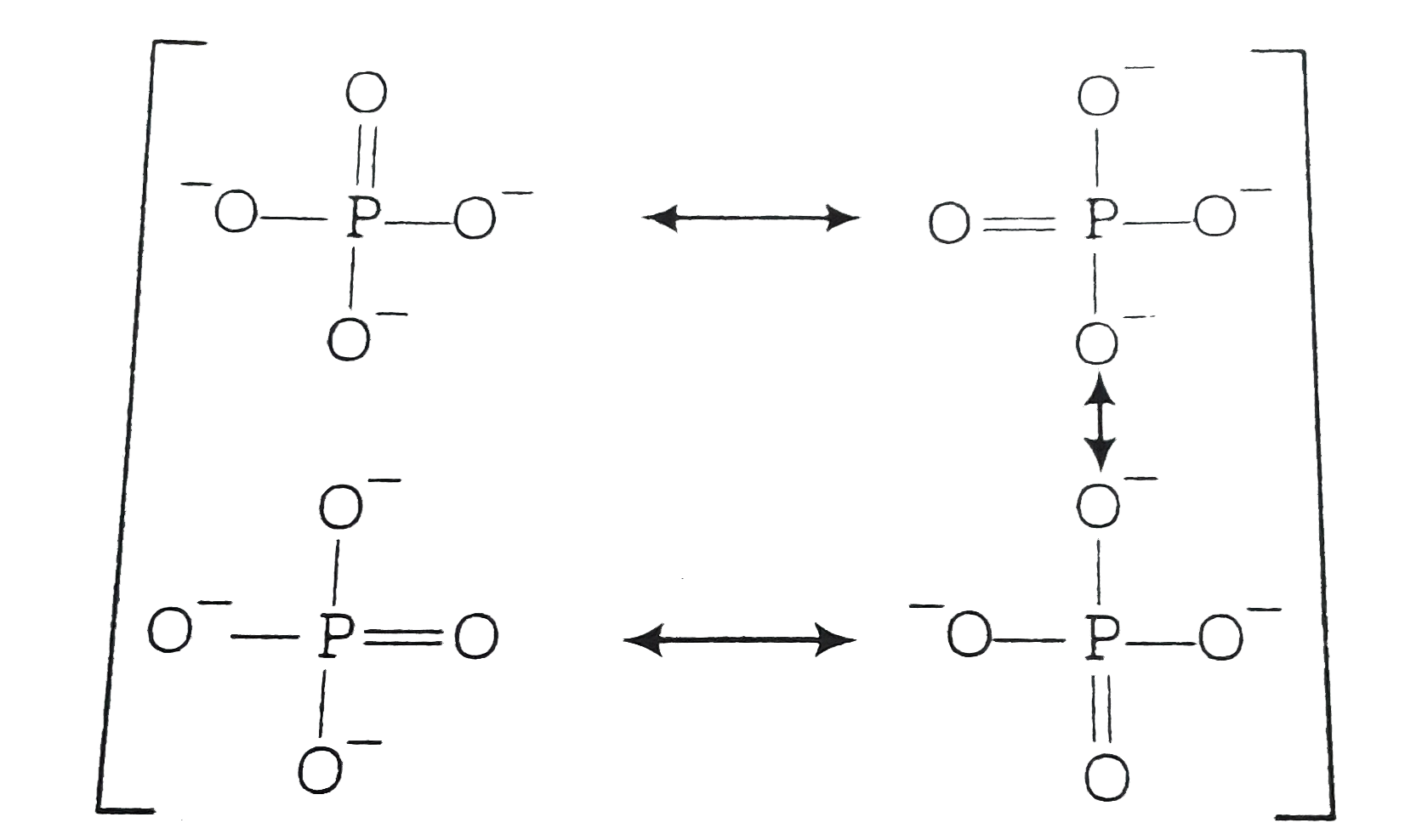
- In PO(4)^(3-) ion, the formal charge on the oxygen atom of P - O bond ...
Text Solution
|
- In PO(4)^(3-) the formal charge on each O-atom and P-O bond order resp...
Text Solution
|
- In PO(4)^(3-) ion the formal charge on the oxygen atom of P-O bond is
Text Solution
|
- In PO(4)^(3-) ion, the formal charge on the oxygen atom of P-O bond is
Text Solution
|
- In PO(4)^(3-) ion the formal charge on the oxygen atom of P-O bond is
Text Solution
|
- In PO(4)^(3-) , the formal change on each oxygen atom and the P-O bond...
Text Solution
|
- In H(3)O^(+) the formal charge on the oxygen atom is :
Text Solution
|
- In PO(4)^(3-) ion, the effective charge on each oxygen atom and P-O bo...
Text Solution
|
- PO(4)^(3-) आयन में प्रत्येक ऑक्सीजन पर सामान्य आवेश तथा P-0 आबन्ध कोटि...
Text Solution
|