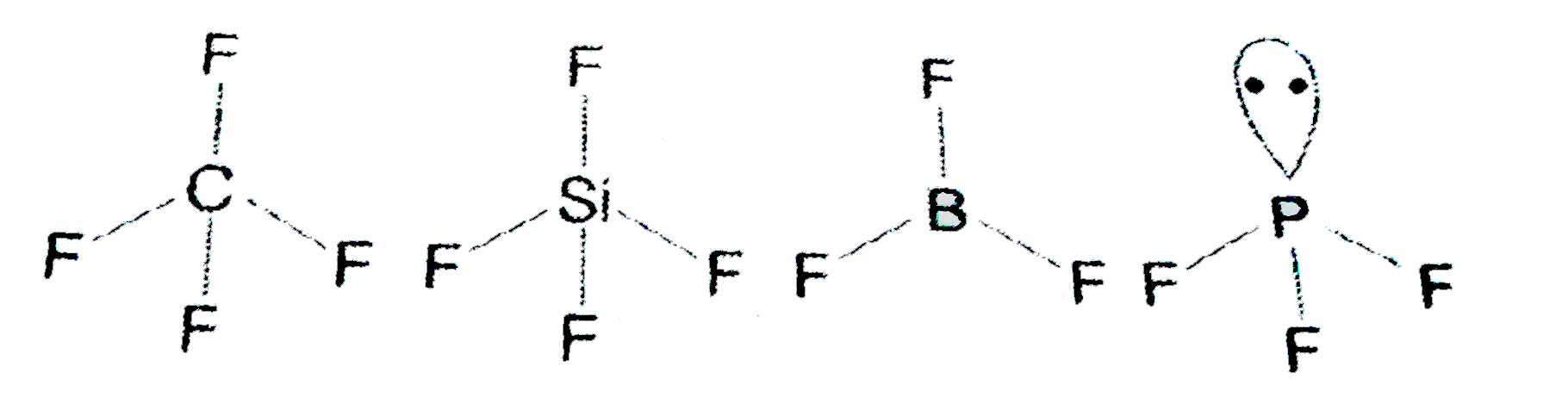
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following fluoro-compound is most likely to behave as a L...
Text Solution
|
- Which of the of the following fluoro -compouds is most likely to beahv...
Text Solution
|
- Which of the following fluoro-compounds is most likely to behave as a ...
Text Solution
|
- Which of the following fluoro-compounds is most likely to behave as a ...
Text Solution
|
- Which of the of the following fluoro -compouds is most likely to beahv...
Text Solution
|
- Which of the following fluoro- compounds is most likely to behave ...
Text Solution
|
- Which of the following fluoro- compounds is most likely to behave as a...
Text Solution
|
- Which of the following fluoro-compounds is most likely to behave as a ...
Text Solution
|
- निम्न में से कौन - सा फ्लुओरो योगिक सर्वाधिक रूप से लुईस क्षार की तरह ...
Text Solution
|