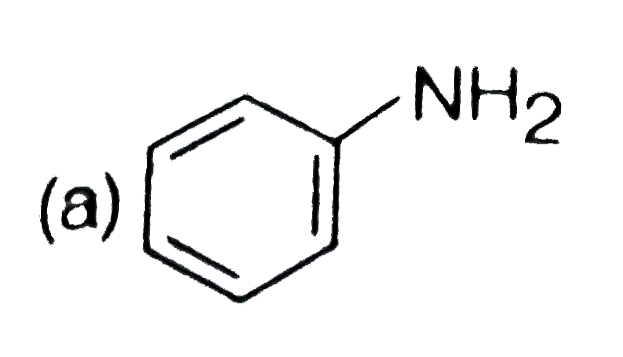
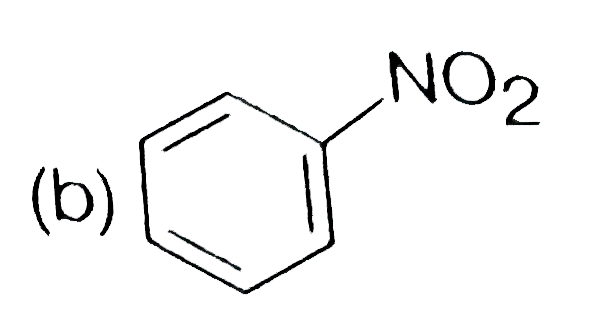
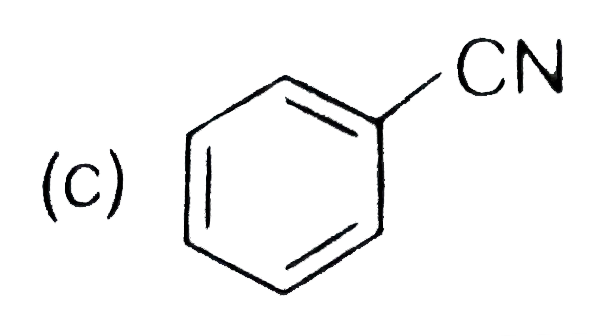
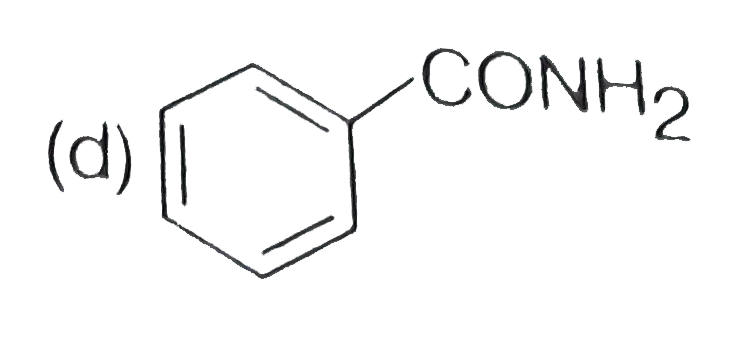
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
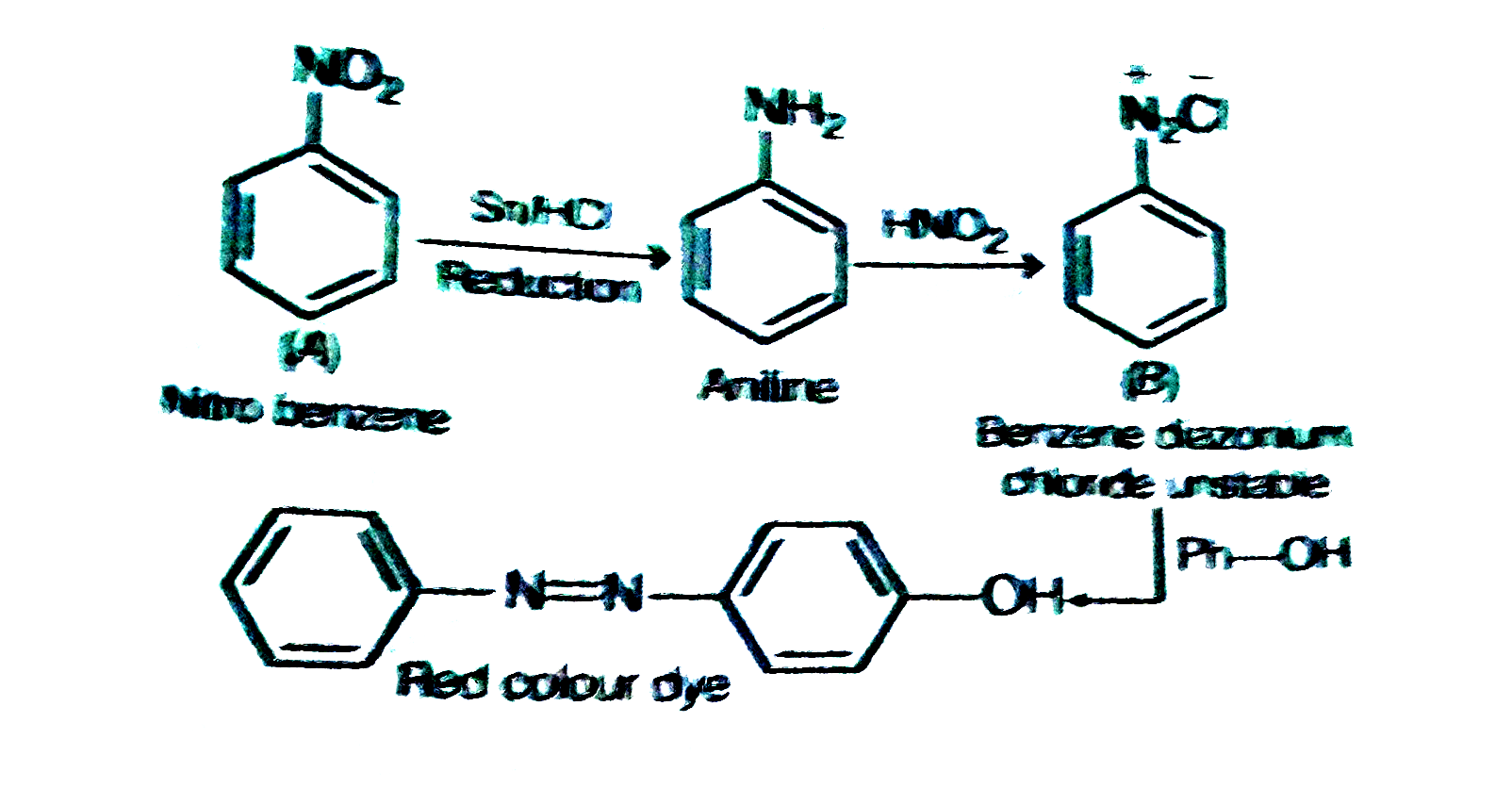
- A given nitrogen-containing compound A reacts with Sn//HCl followed by...
Text Solution
|
- A given nitrogen-containing compound A reacts with Sn//HCI followed by...
Text Solution
|
- A given nitrogen-containing compound A reacts with Sn//HCI followed by...
Text Solution
|
- A given nitrogen-containing aromatic compound A reacts with Sn//HCl, f...
Text Solution
|
- A given nirogen containing aromatic compound 'A' reacts with Sn/HCl, f...
Text Solution
|
- Compound (A) with molecular formula C(2)H(4)O reduces Tollen's reagent...
Text Solution
|
- A nitrogen -containing aromatic compound A reacts with Sn/HCl, followe...
Text Solution
|
- एक दिया गया नाइट्रोजन युक्त A,Sn//HCl तथा बाद मे HNO(2) से क्रिया करके...
Text Solution
|
- एक दिया गया नाइट्रोजन -युक्त ऐरोमेटिक यौगिक (A )Sn /HCI ,तत्पश्चात HNO...
Text Solution
|