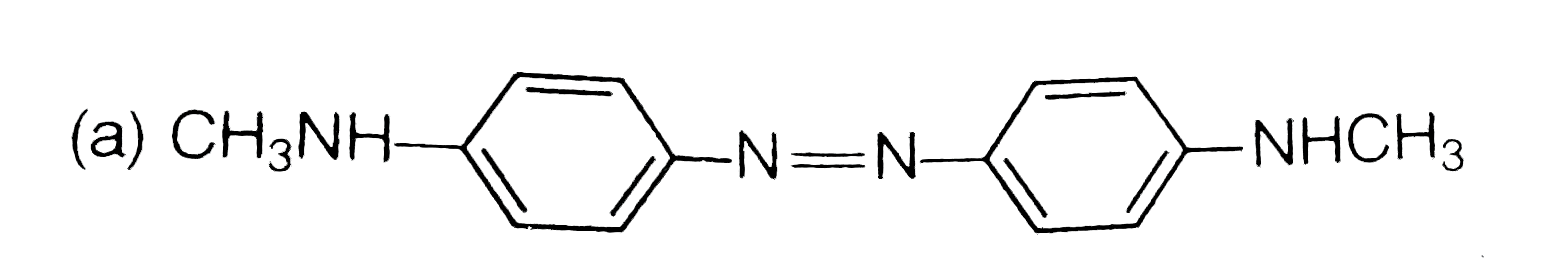
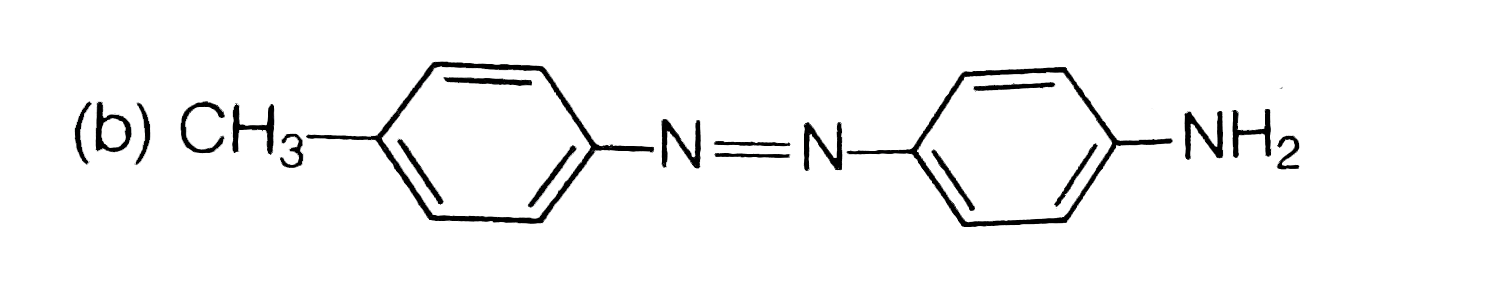
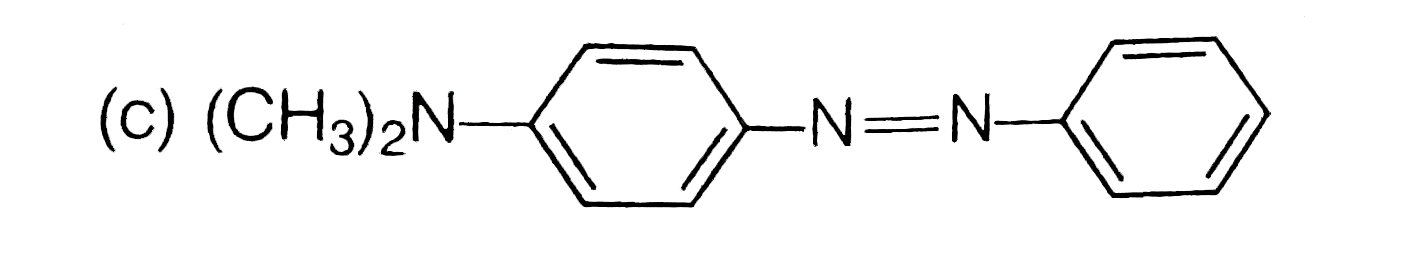
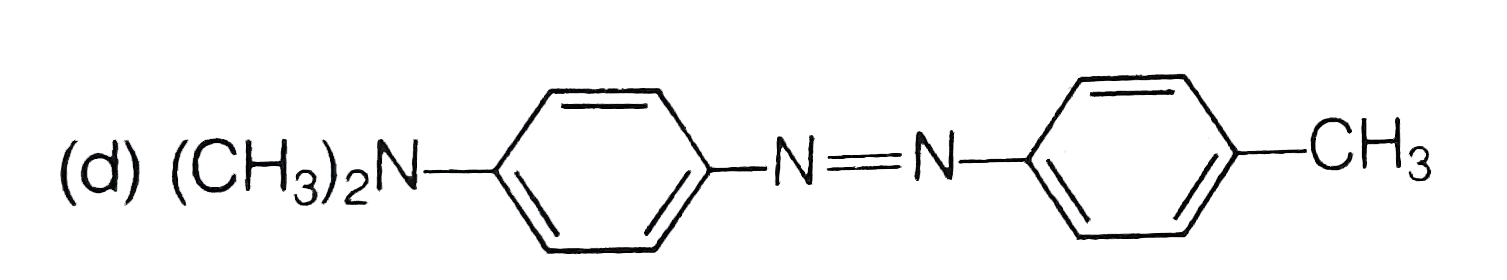
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Aniline when diazotized in cold and then treated with dimethyl aniline...
Text Solution
|
- Aniline when treated with conc. HNO3 gives
Text Solution
|
- Benzene diazonium chloride is the product when aniline is treated with
Text Solution
|
- In the reaction of aniline, a coloured product C was obtained. The str...
Text Solution
|
- Dimethyl ether when heated with exces HI gives :
Text Solution
|
- Aniline when diazontized in cold and then trated with aniline gives co...
Text Solution
|
- Diazotization of aniline gives
Text Solution
|
- When aniline is treated with CHCl(3) and KOH, the product is
Text Solution
|
- ऐनिलीन को जब ठण्डे में डाइऐजोटीकृत करके तथा फिर N N - डाइमेथिलऐनिलीन क...
Text Solution
|