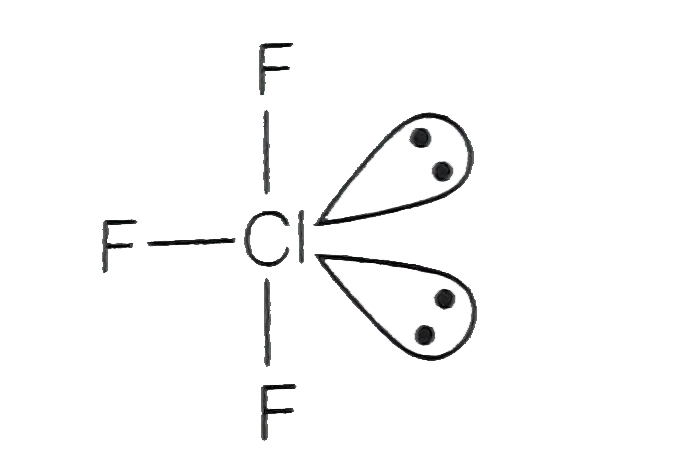
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of lone pair of electrons on each atom of oxygen molecule i...
Text Solution
|
- The number of lone pairs of electrons on xenon atom in XeF(4) molecule...
Text Solution
|
- The number of electrons that are paired in oxygen molecule is
Text Solution
|
- The number of lone pair of electrons in central atom of CIF5
Text Solution
|
- The number of bond pair and lone pair on oxygen atom in ether are resp...
Text Solution
|
- The number of electrons that are paired in oxygen molecule is
Text Solution
|
- Number of lone pairs and bond pairs present on oxygen atom in water a...
Text Solution
|
- Number of lone pairs of electrons in P(4) molecule = …….. .
Text Solution
|
- Molecule having maximum number of lone pairs of electrons on central a...
Text Solution
|