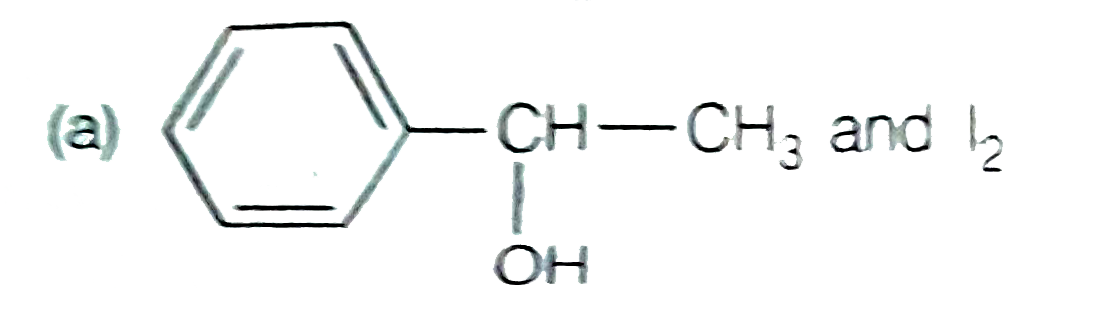
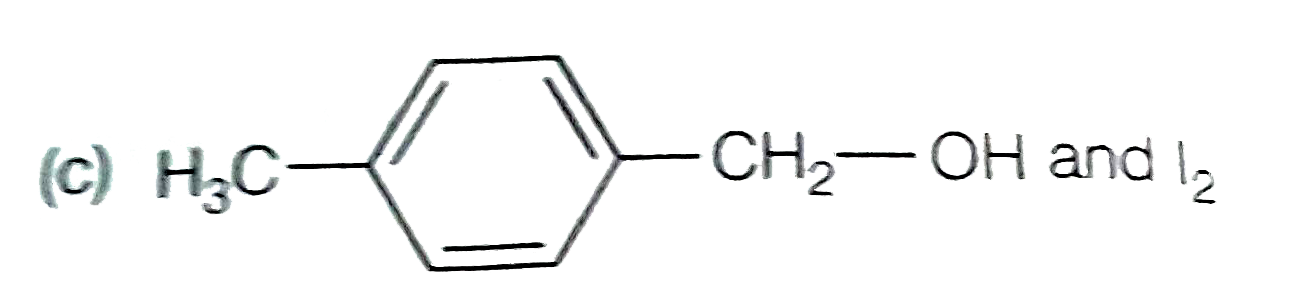
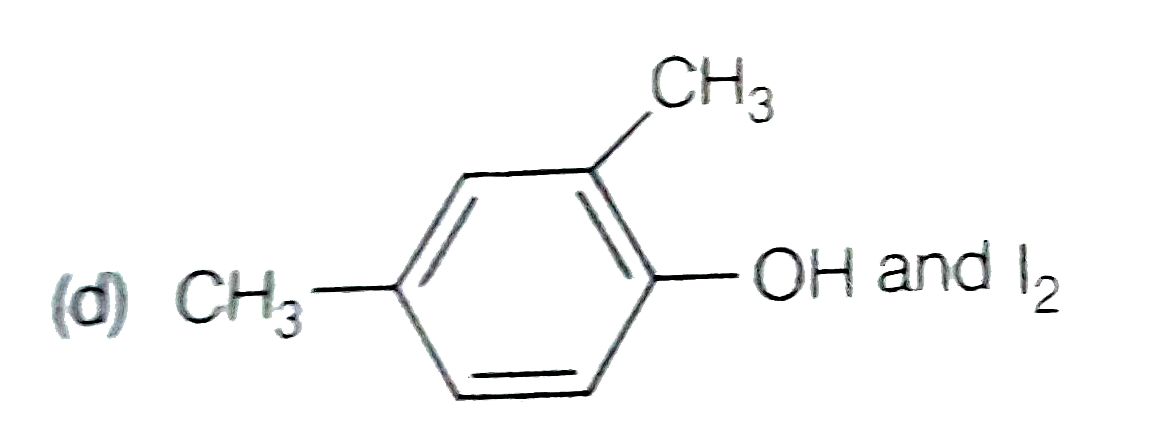
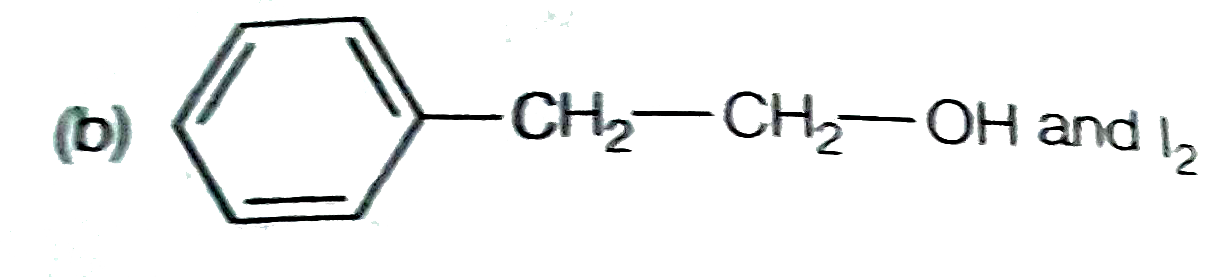A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
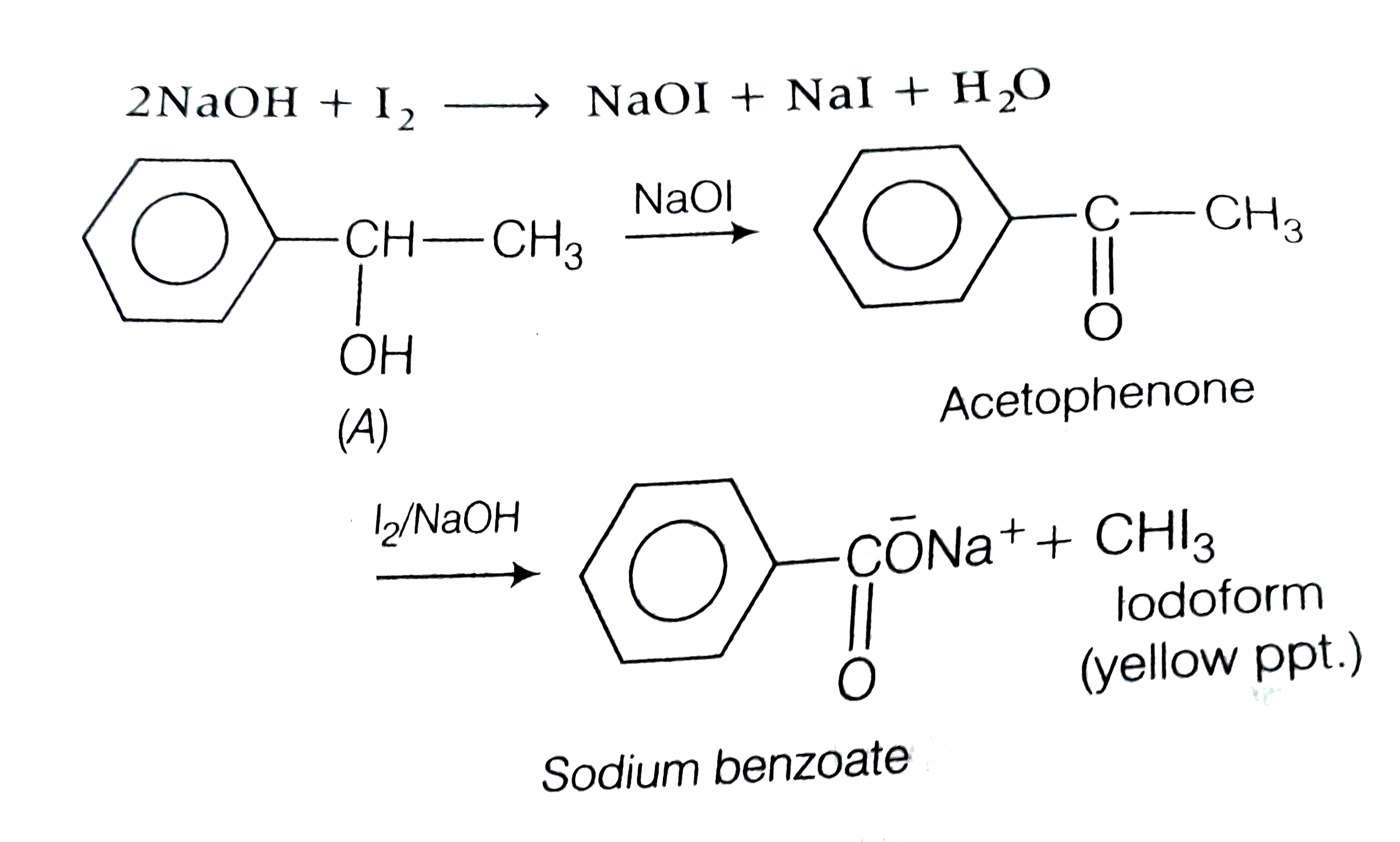
- Compound A,C(8)H(10)O, is found to react with NaOI (produced by reacti...
Text Solution
|
- Compound A,C(8)H(10)O , id found to react with NaOI (produced by react...
Text Solution
|
- An aromatic compound A of the molecular formula C(8)H(10)O on reaction...
Text Solution
|
- X' compound (C(4)H(8)O) decolorises bromine water react with I(2) & Na...
Text Solution
|
- Compound A, C(8)H(10)O , is found to react with NaOl (Produced by reat...
Text Solution
|
- One mole of an organic compound (A) having M.F. C(2)H(6)O reacts with ...
Text Solution
|
- Compound A, C(8)H(10)O , is found to react with NaOH (produced by reac...
Text Solution
|
- यौगिक A, (C8H10 O), NaOl (NaOH के साथ Y की अभिक्रिया द्वारा उत्पादित) ...
Text Solution
|
- Compound A, C(8)H(10)O is found to react with NaOI (produced by reacti...
Text Solution
|