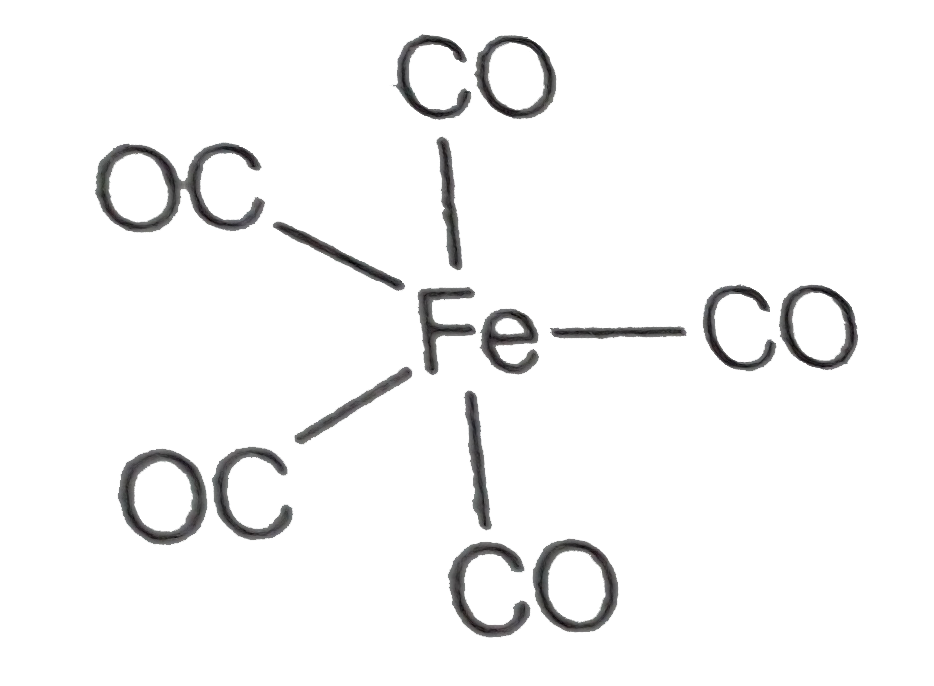
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Iron carbonyl, Fe(CO)5 is
Text Solution
|
- Iron carbonyl, Fe(CO)5 is
Text Solution
|
- आयरन कार्बोनिल, Fe(CO)(5) है-
Text Solution
|
- Iron carbonyl, Fe(CO)(5) is -
Text Solution
|
- Iron carbonyl, Fe(CO)(5) is -
Text Solution
|
- Cordination no. Of [Fe(CO)5] is
Text Solution
|
- Cordination no. Of [Fe(CO)5] is
Text Solution
|
- Cordination no. Of [Fe(CO)5] is
Text Solution
|
- Cordination no. Of [Fe(CO)5] is
Text Solution
|