Consider the change in oxidation state of Bromine corredponding to different emf values as shown in the diagram below :
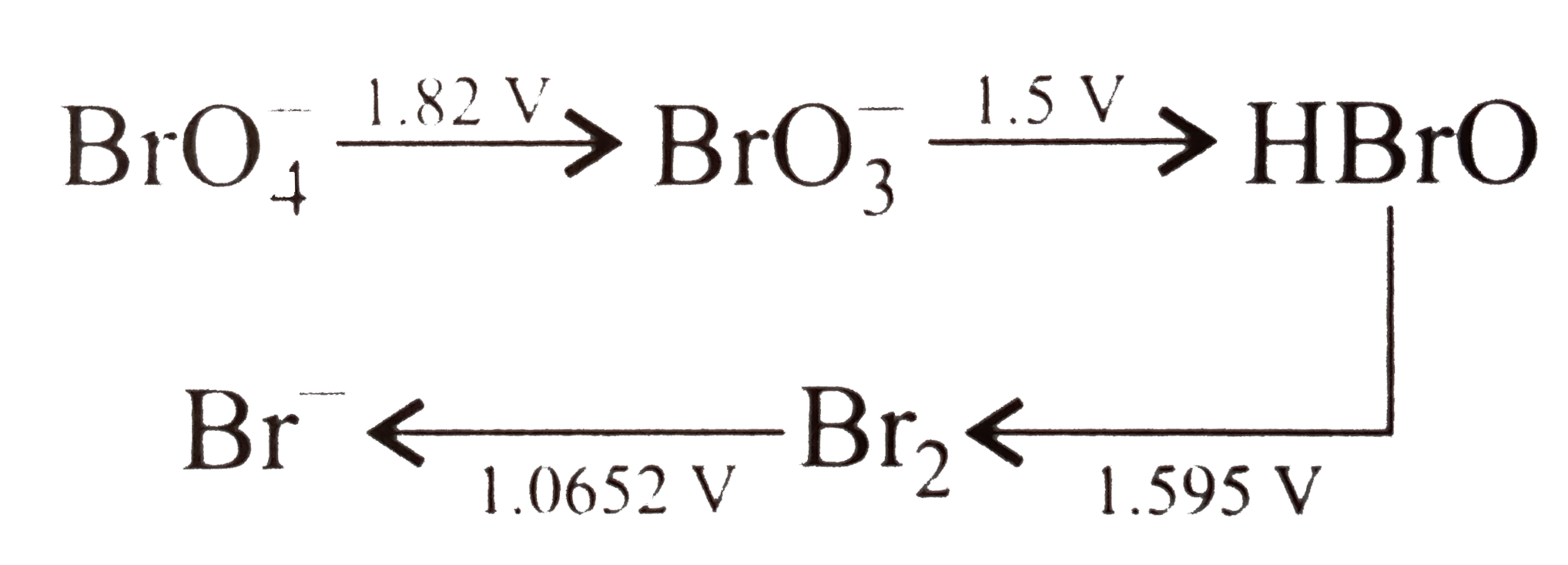 .
.
The species undergoing disproportionation is .
Consider the change in oxidation state of Bromine corredponding to different emf values as shown in the diagram below :
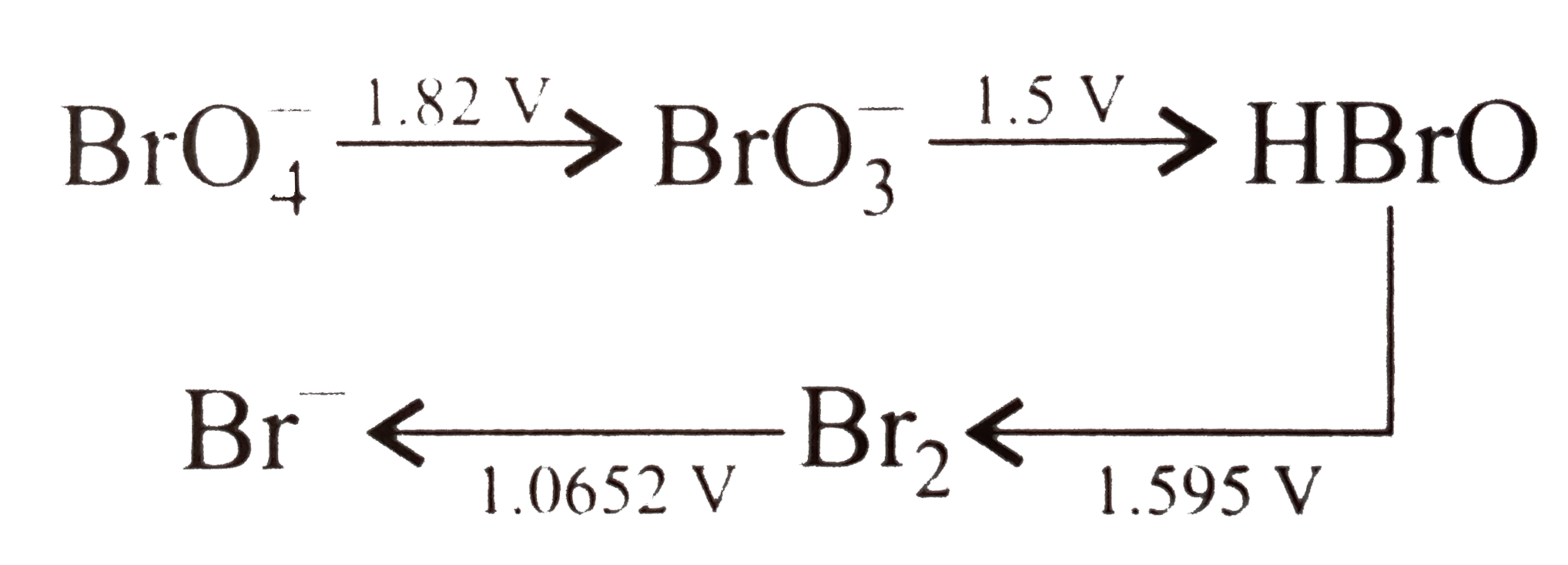 .
.
The species undergoing disproportionation is .
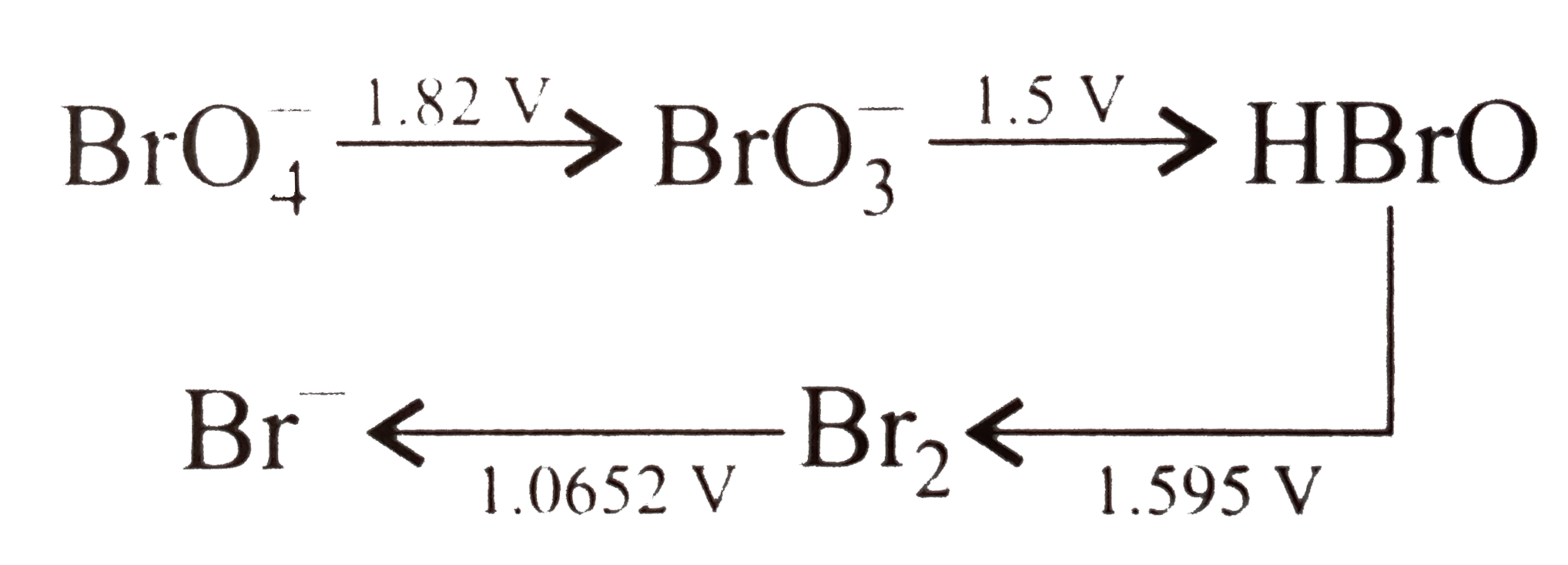 .
. The species undergoing disproportionation is .
A
`Br_(2)`
B
`BrO_(4)^(-)`
C
`BrO_(3)^(-)`
D
`HBrO`
Text Solution
Verified by Experts
The correct Answer is:
D
Key Concept The reaction in which same species is oxidised as well as reduced is called disproportionation reaction. Firstly, calculate the value of `E_("cell")^(@)` of each species undergoing disproportionation reaction. The reaction whose `E_("cell")^(@)` value is positive will be feasible (spontaneous).
(i) Given, `BrO_(3)^(-) to HBrO, E_(BrO_(3)^(-)//HBrO)=1.5V`
`BrO_(3)^(-) to BrO_(4)^(-), E_(BrO_(3)^(-)//BrO_(4)^(-))=-1.82V`
`therefore 2overset(+5)(BrO_(3)^(-)) to overset(+1)(HBrO)+overset(+7)(B)rO_(4)^(-)`
`E_("cell")^(@)=E_("red")^(@)+E_(ox)^(@)`
`E_(BrO_(3)^(-)//HBrO)^(@)+E_(BrO_(3)^(-)//BrO_(4)^(-))^(@)`
`=1.5-1.82=-0.32 V`
`(ii) overset(+1)(HBrO) to overset(0)(Br_(2)), E_(HBrO//Br_(2))^(@)=1.595 V`
`overset(+1)(HBrO)to overset(+5)(BrO_(3)^(-)), E_(HBrO//BrO_(3)^(-))^(@)=-1.5V`
`overset(+1)(2HBrO)to overset(0)(Br_(2))+overset(+5)(BrO_(3)^(-))`
`E_("cell")^(@)=E_(Br_(2)//Br^(-))+^(@)+E_(Br_(2)//HBrO)^(@)`
`=1.0652-1.595=-0.5298V`
`therefore` Among the given options, only HBrO undergoes disproportionation.
(i) Given, `BrO_(3)^(-) to HBrO, E_(BrO_(3)^(-)//HBrO)=1.5V`
`BrO_(3)^(-) to BrO_(4)^(-), E_(BrO_(3)^(-)//BrO_(4)^(-))=-1.82V`
`therefore 2overset(+5)(BrO_(3)^(-)) to overset(+1)(HBrO)+overset(+7)(B)rO_(4)^(-)`
`E_("cell")^(@)=E_("red")^(@)+E_(ox)^(@)`
`E_(BrO_(3)^(-)//HBrO)^(@)+E_(BrO_(3)^(-)//BrO_(4)^(-))^(@)`
`=1.5-1.82=-0.32 V`
`(ii) overset(+1)(HBrO) to overset(0)(Br_(2)), E_(HBrO//Br_(2))^(@)=1.595 V`
`overset(+1)(HBrO)to overset(+5)(BrO_(3)^(-)), E_(HBrO//BrO_(3)^(-))^(@)=-1.5V`
`overset(+1)(2HBrO)to overset(0)(Br_(2))+overset(+5)(BrO_(3)^(-))`
`E_("cell")^(@)=E_(Br_(2)//Br^(-))+^(@)+E_(Br_(2)//HBrO)^(@)`
`=1.0652-1.595=-0.5298V`
`therefore` Among the given options, only HBrO undergoes disproportionation.
Similar Questions
Explore conceptually related problems
Consider the change in oxidation state of Bromine corredponding to different emf values as shown in the diagram below : . The the species undergoing dispropprtionation is .
Consider the change in oxidation state of Bromine corredponding to different emf values as shown in the diagram below : . The the species undergoing dispropprtionation is .
In which of the following compounds oxidation state of chlorine has two different values?
How can we show that change in oxidation state is different from variable oxidation state for non transition element ?
An ideal gas undergoing a change of state from A to B through four different paths I, II,III and IV as shown in the P-V diagram that lead to the same change of state tyhen the change in internal energy is
If an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is CIO_(4)^(-)overset(+1.20V)toCiO_(3)^(-)overset(+1.18V)toHClO_(2)overset(+1.60V)toHClOoverset(1.67V)toCl_(2)overset(1.36V)toCl^(-) in basic solution. ClO_(4)^(-)overset(0.37V)toClO_(3)^(-)overset(0.30V)toClO_(2)^(-)overset(0.68V)toClO^(-)overset(0.42V)toCl_(2)overset(1.36V)toCl^(-) The standard potentials for two nonadjacent species can also be calculateed by using the concept that triangleG^(@) as an additive property but potential is not an additive property and triangleG^(@)=-nFx^(0) . if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of triangleG^(@)//F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species. Q. For a hypothetical element, the frost diagram is shown in figure.? which of the following oxidation state is least stable?
If an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is CIO_(4)^(-)overset(+1.20V)toCiO_(3)^(-)overset(+1.18V)toHClO_(2)overset(+1.60V)toHClOoverset(1.67V)toCl_(2)overset(1.36V)toCl^(-) in basic solution. ClO_(4)^(-)overset(0.37V)toClO_(3)^(-)overset(0.30V)toClO_(2)^(-)overset(0.68V)toClO^(-)overset(0.42V)toCl_(2)overset(1.36V)toCl^(-) The standard potentials for two nonadjacent species can also be calculateed by using the concept that triangleG^(@) as an additive property but potential is not an additive property and triangleG^(@)=-nFx^(0) . if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of triangleG^(@)//F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species. Q. What is the potential couple (ClO^(-))/(Cl^(-)) at pH=14 ?
If an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is CIO_(4)^(-)overset(+1.20V)toCiO_(3)^(-)overset(+1.18V)toHClO_(2)overset(+1.60V)toHClOoverset(1.67V)toCl_(2)overset(1.36V)toCl^(-) in basic solution. ClO_(4)^(-)overset(0.37V)toClO_(3)^(-)overset(0.30V)toClO_(2)^(-)overset(0.68V)toClO^(-)overset(0.42V)toCl_(2)overset(1.36V)toCl^(-) The standard potentials for two nonadjacent species can also be calculateed by using the concept that triangleG^(@) as an additive property but potential is not an additive property and triangleG^(@)=-nFx^(0) . if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of triangleG^(@)//F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species. Q. Which of the following statement is correct?
A cell of emf 2 V and internal resistance 1.2Omega is connected with an ammeter of resistance 0.8Omega and two resistors of 4.5Omegaand9Omega as shown in the diagram below: What is the potential difference across the terminals of the cell ?
An element has the minimum and maximum oxidation states as -X and +X respectively. It does not have the possibility of undergoing disproportionation in any of its compounds. What is the value of X ?
Recommended Questions
- Consider the change in oxidation state of Bromine corredponding to dif...
Text Solution
|
- Consider the change in oxidation state of Bromine corredponding to dif...
Text Solution
|
- The species that undergoes disproportionation is an alkaline medium ar...
Text Solution
|
- Consider the change in oxidation state of bromine corresponding to dif...
Text Solution
|
- Consider the change in oxidation state of bromide corresponding to dif...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to dif...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to di...
Text Solution
|
- Consider the change in the oxidation states of bromine depending on th...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to dif...
Text Solution
|