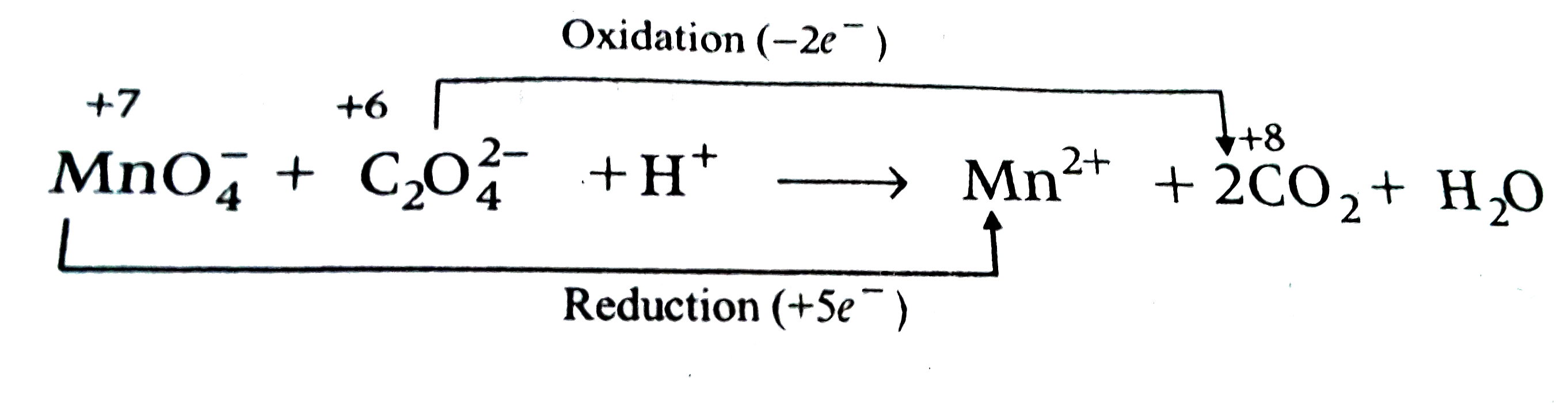A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the redox reaction xMnO(4)^(ө)+yC(2)O(4)^(2-)+zH^(o+)rarrMn^(2+)...
Text Solution
|
- For the redox reaction MnO(4)^(ө)+C(2)O(4)^(2-)+H^(o+)rarrMn^(2+)+CO...
Text Solution
|
- MnO(4)^(-) oxidises H(2)O(2) to O(2) in acidic medium xMnO(4)^(-)+yH...
Text Solution
|
- Consider the following reaction : xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+) to...
Text Solution
|
- For redox reaction {:(xMnO(4)^(-)+yH(2)C(2)O(4)+ZH^(+)),(" "darr),(mMn...
Text Solution
|
- निम्नलिखित अभिक्रिया पर विचार कीजिये: xMnO(4)^(-)+yC(2)O(4)^(2-)+zH...
Text Solution
|
- Consider the following reaction: xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+) t...
Text Solution
|
- Consider the following reaction : xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+)...
Text Solution
|
- Consider the following reaction xMnO(4)^(-)+yC(2)IO(4)^(2-)+zH^(+)rarr...
Text Solution
|