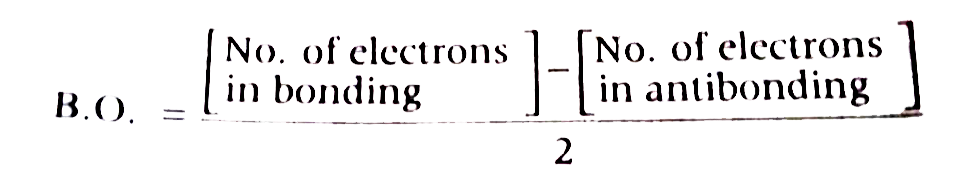A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following species : CN^(+) ,CN^(-) ,NO and CN Which...
Text Solution
|
- State the bond order and indicate whether the species is paramagnetic ...
Text Solution
|
- Consider the following species CN^(-),CN^(-),NO and CN . Which one of ...
Text Solution
|
- What is the bond order of CN^(-) specie?
Text Solution
|
- Write of the following have identical bond order ? CN^(-), O(2)^(-) , ...
Text Solution
|
- Consider the following species : CN^(+), CN^(-), NO and CN Which one o...
Text Solution
|
- Consider the following species, CN^(+),CN^(-)NO and CN which one of th...
Text Solution
|
- Consider the following species : CN^(+) , CN^(-) , NO and CN Which one...
Text Solution
|
- CN^(+),CN^(-),NO,CN, सर्वाधिक बन्ध क्रम किसका है ?
Text Solution
|