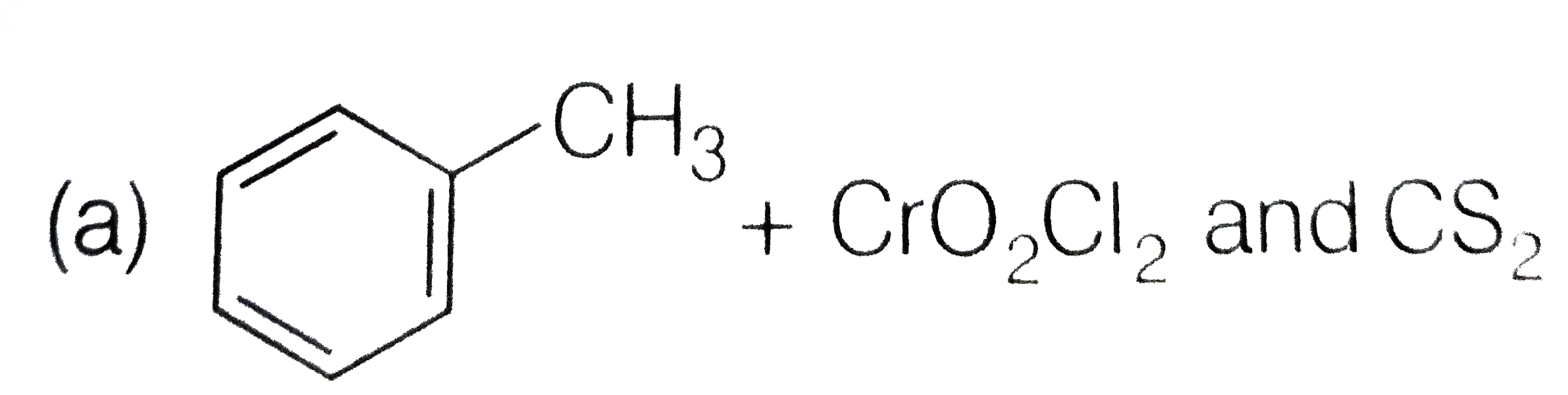
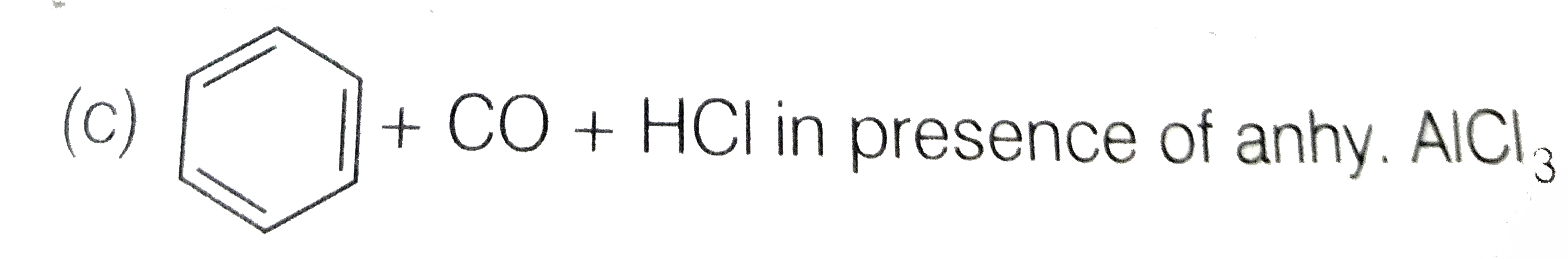
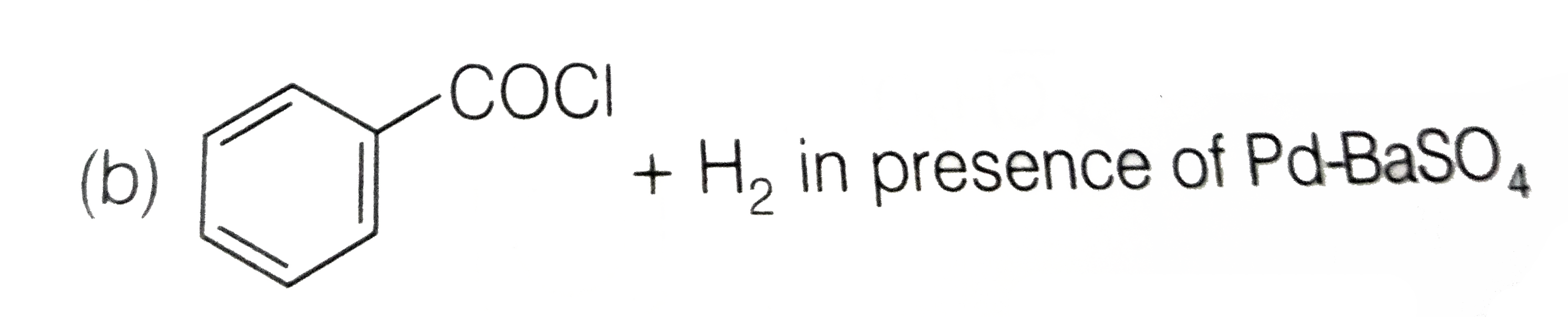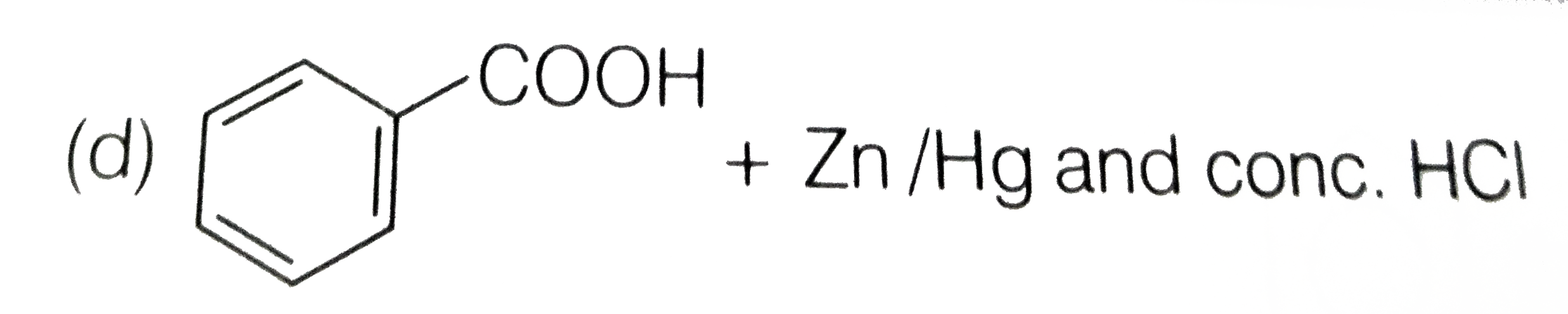A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Reaction by which benzaldehyde cannot be prepared is :
Text Solution
|
- Cinnamic acid form benzaldehyde would be prepared by which of the foll...
Text Solution
|
- Reaction by which benzaldehyde cannot be prepared is :
Text Solution
|
- Reaction by which Benzaldehyde cannot be prepared
Text Solution
|
- Reaction by which benzaldehyde cannot be prepared is :
Text Solution
|
- Reaction by which benzaldehyde cannot be prepared , is
Text Solution
|
- The reaction by which Benzaldehyde can not be prepared
Text Solution
|
- How is benzaldehyde prepared by Etard's reaction?
Text Solution
|
- Reaction by which benzaldehyde cannot be prepared
Text Solution
|