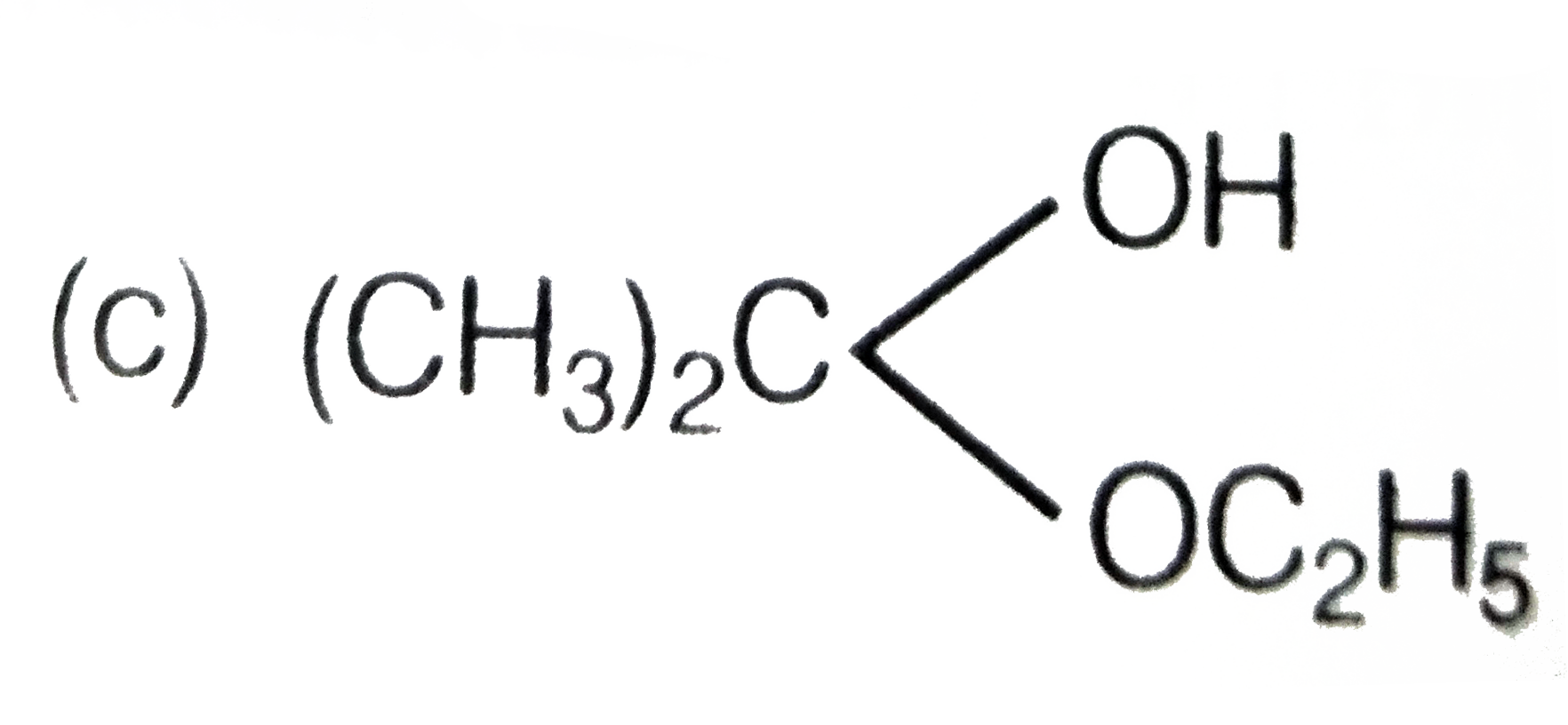
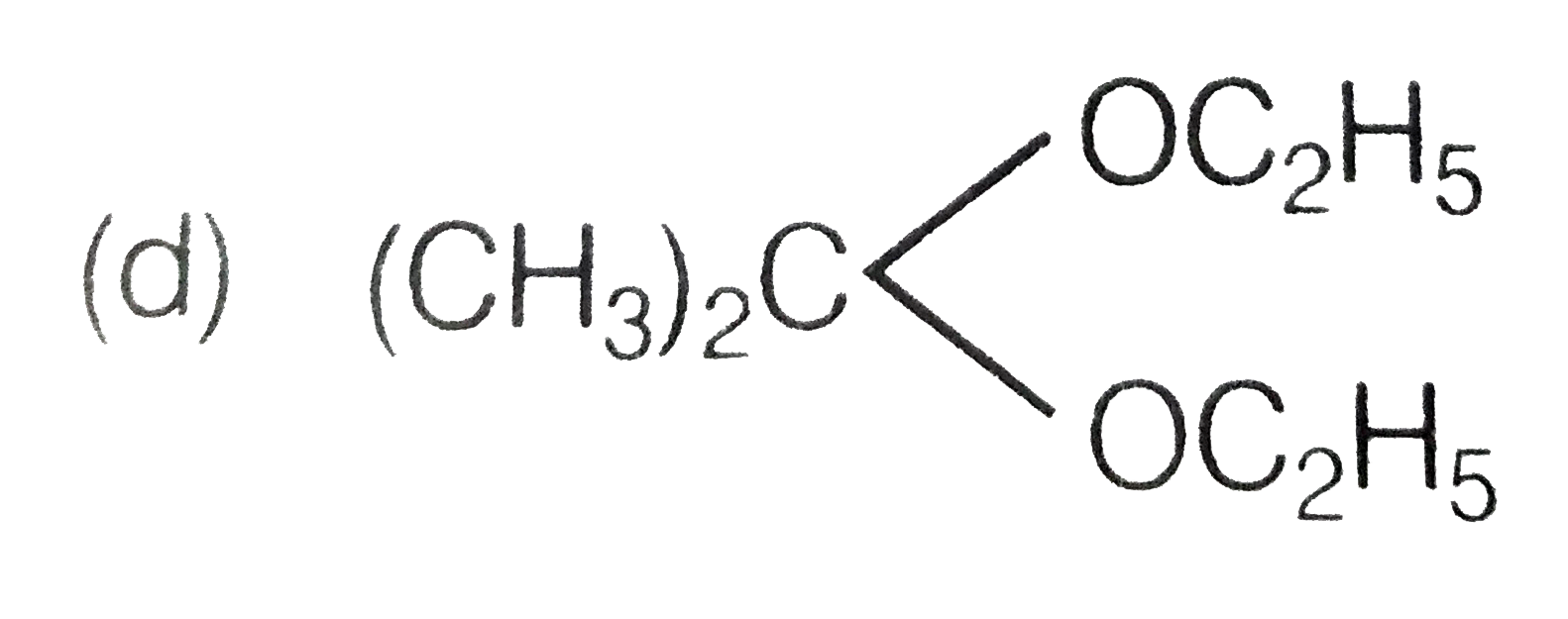A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Acetone is treated with excess of ethanol in the presence of hydrochlo...
Text Solution
|
- The product formed by the reaction of acetaldehyde with excess of etha...
Text Solution
|
- Acetone is treated with excess of ethanol in the presence of hydrochlo...
Text Solution
|
- The product formed with nitromethane is treated with acetone in the pr...
Text Solution
|
- The mass of CO, obtained when 60 g of calcium carbonate is treated wit...
Text Solution
|
- Acetone is treated with excess of ethanol in the presence of hydrochlo...
Text Solution
|
- How is acetone obtained from ethanol? Or How is acetic acid converted ...
Text Solution
|
- Acetone is treated with excess of ethanol in the presence of hydrochlo...
Text Solution
|
- जब ऐसीटिलीन मक्क्यूरिक क्लोराइड की उपस्थिति में हाइड्रोक्लोरिक अम्ल से...
Text Solution
|