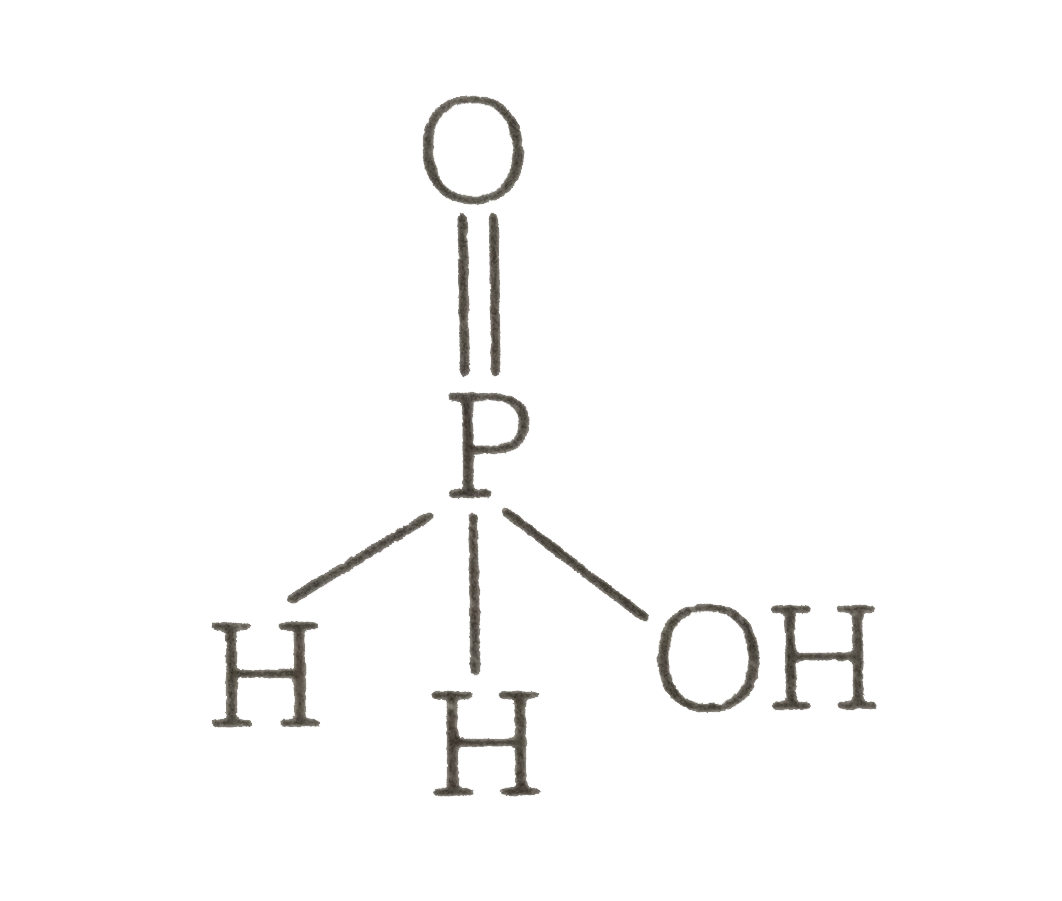
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to:
Text Solution
|
- Strong reducing behaviour of H(3)PO(4) is due to :
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- How do you account of the reducing behaviour for H(3)PO(2) ?
Text Solution
|
- H(3)PO(2) के प्रबल अपचायक गुण का कारण है-
Text Solution
|