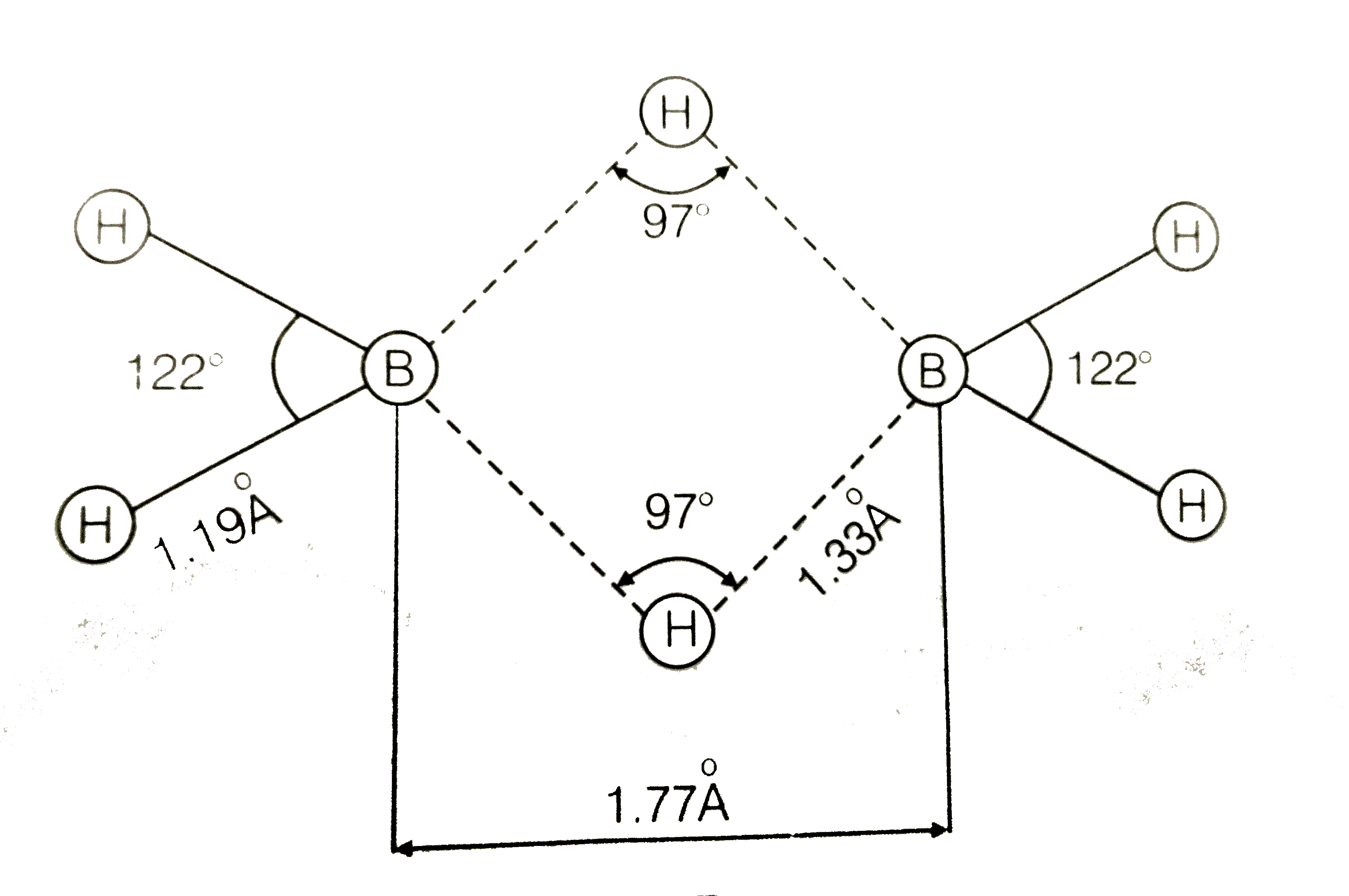
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following hydrides is electron deficient ?
Text Solution
|
- The hydride that is NOT electron deficient is :
Text Solution
|
- प्रत्येक प्रकार के हाइड्राइड के दो उदाहरण लिखिए- (a) इलेक्ट्रॉन परिश...
Text Solution
|
- Which of the following hydrides is electron deficient ?
Text Solution
|
- Explain the following with suitable examples : Electron deficient hy...
Text Solution
|
- Give an example of electron deficient covalent hydride.
Text Solution
|
- Electron-deficient hydride is/are
Text Solution
|
- Which one of the following is an electron deficient hydride?
Text Solution
|
- Electron deficient molecular hydride is
Text Solution
|