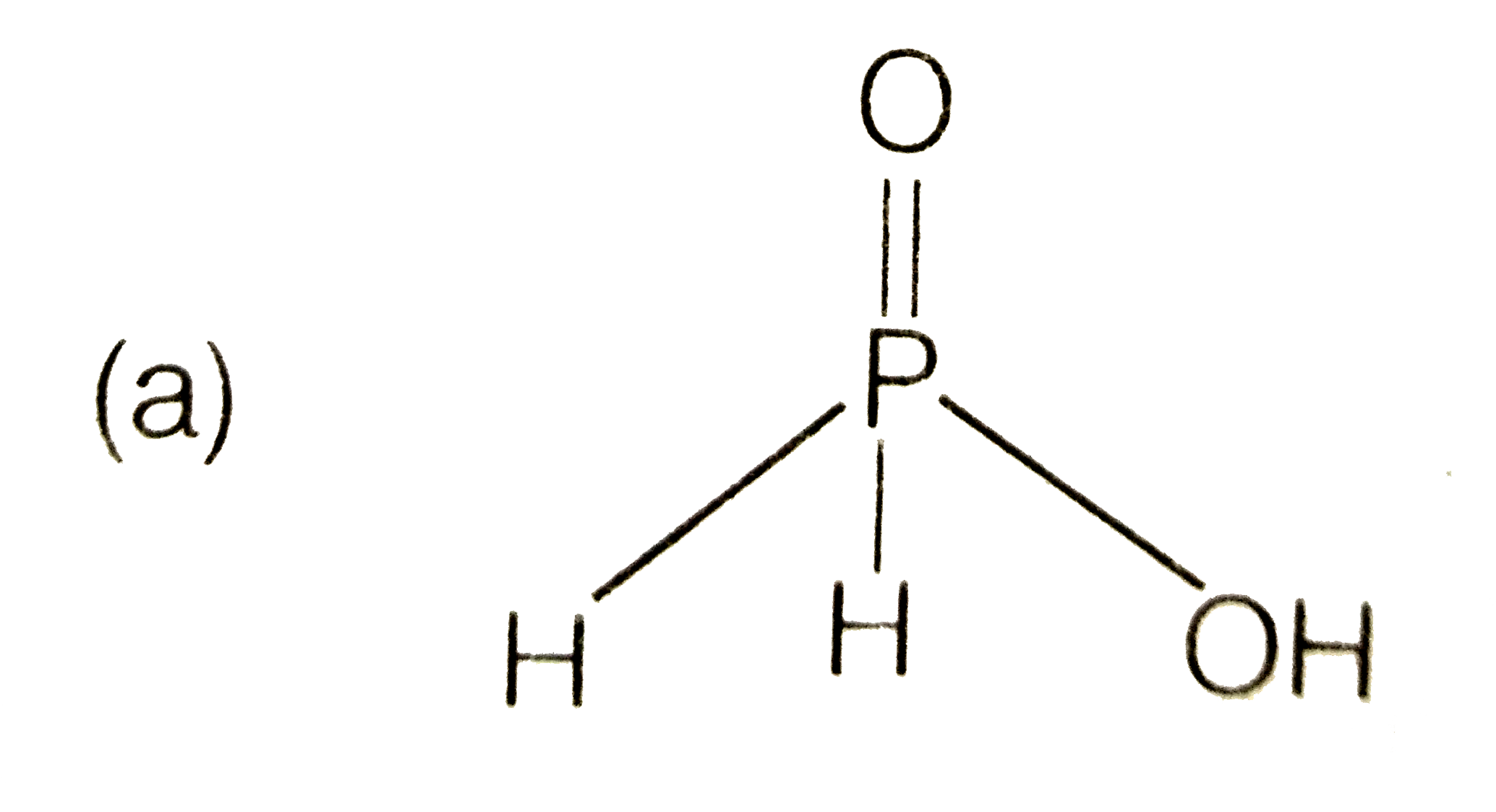
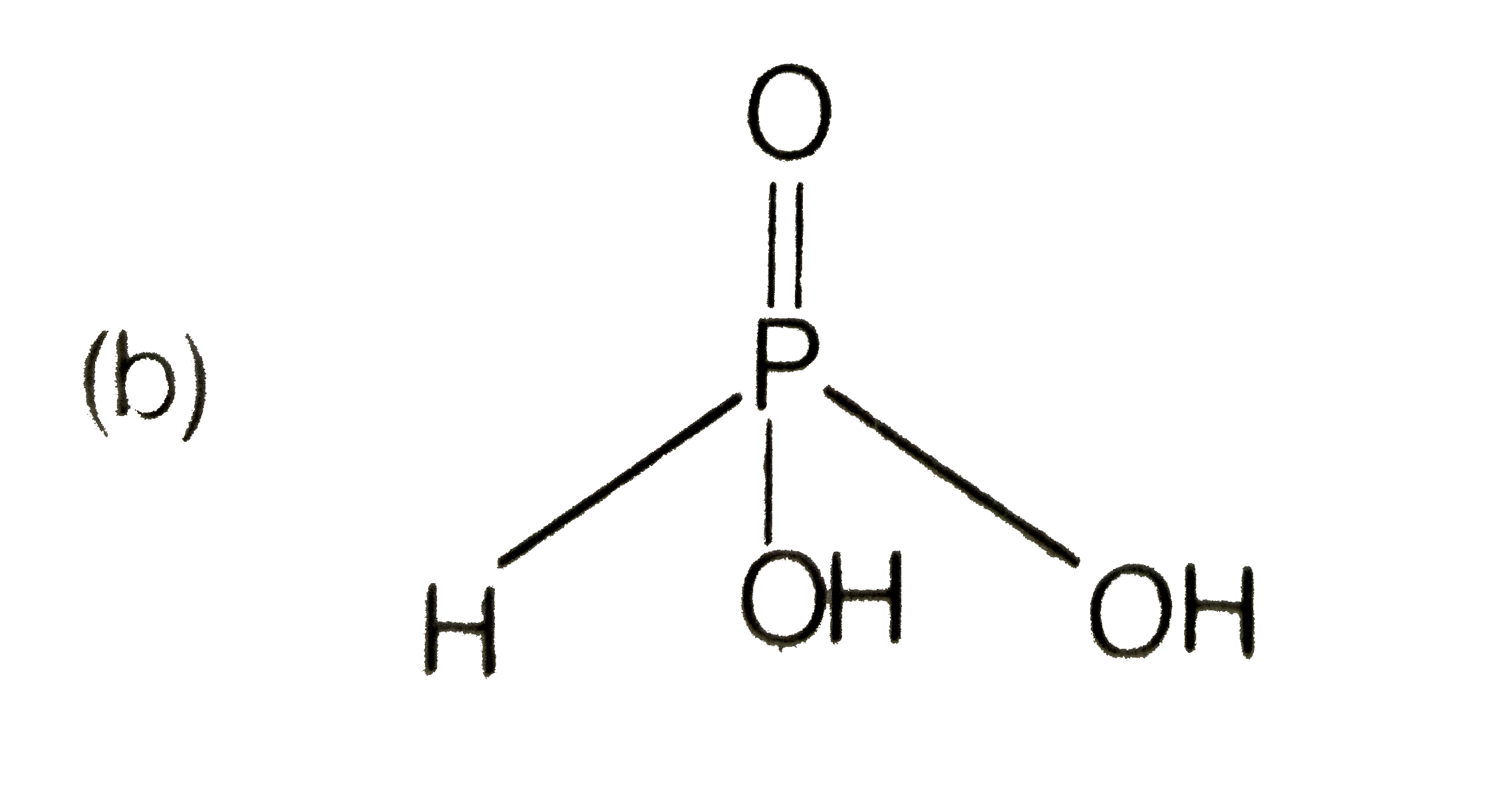
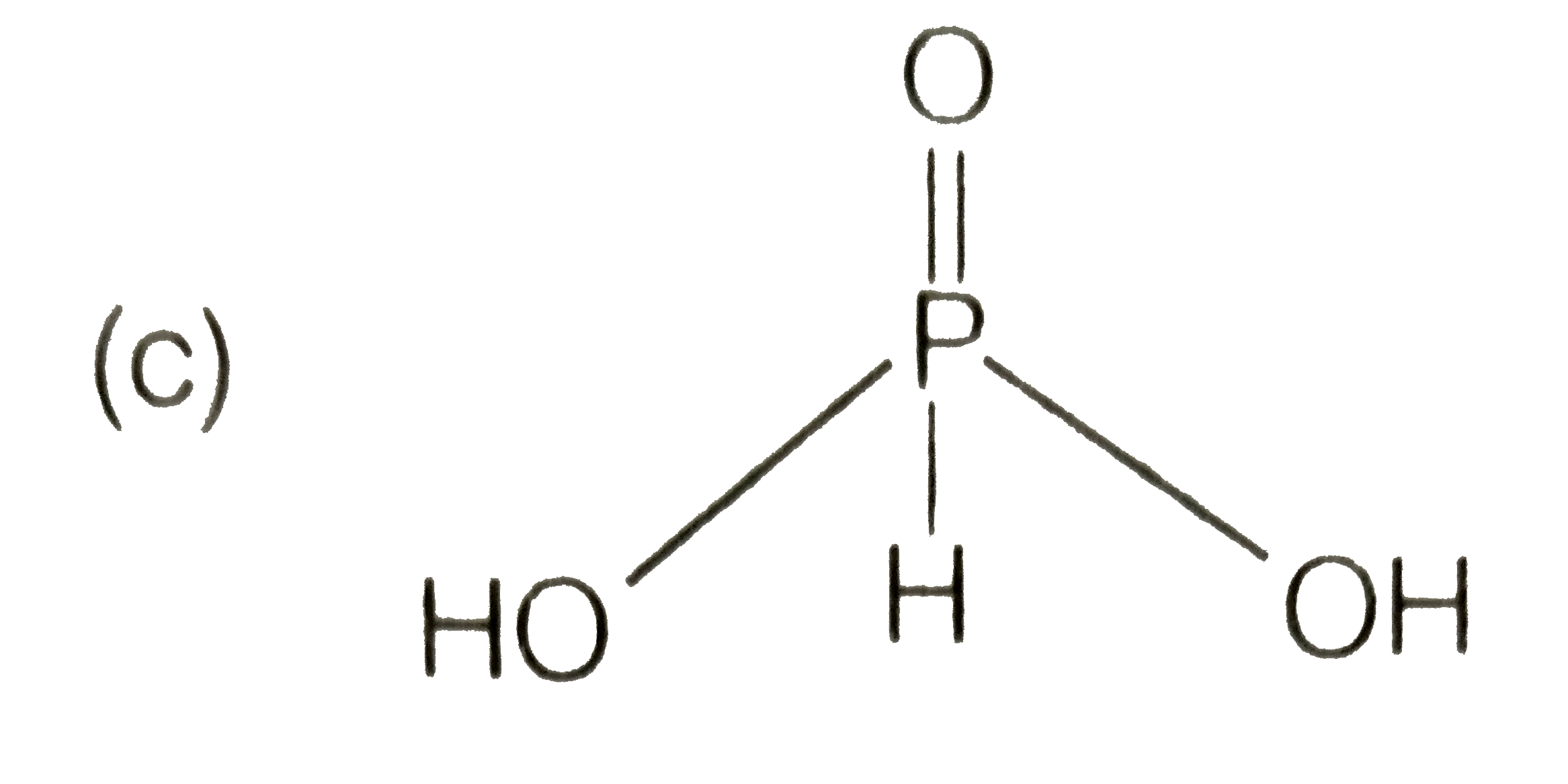
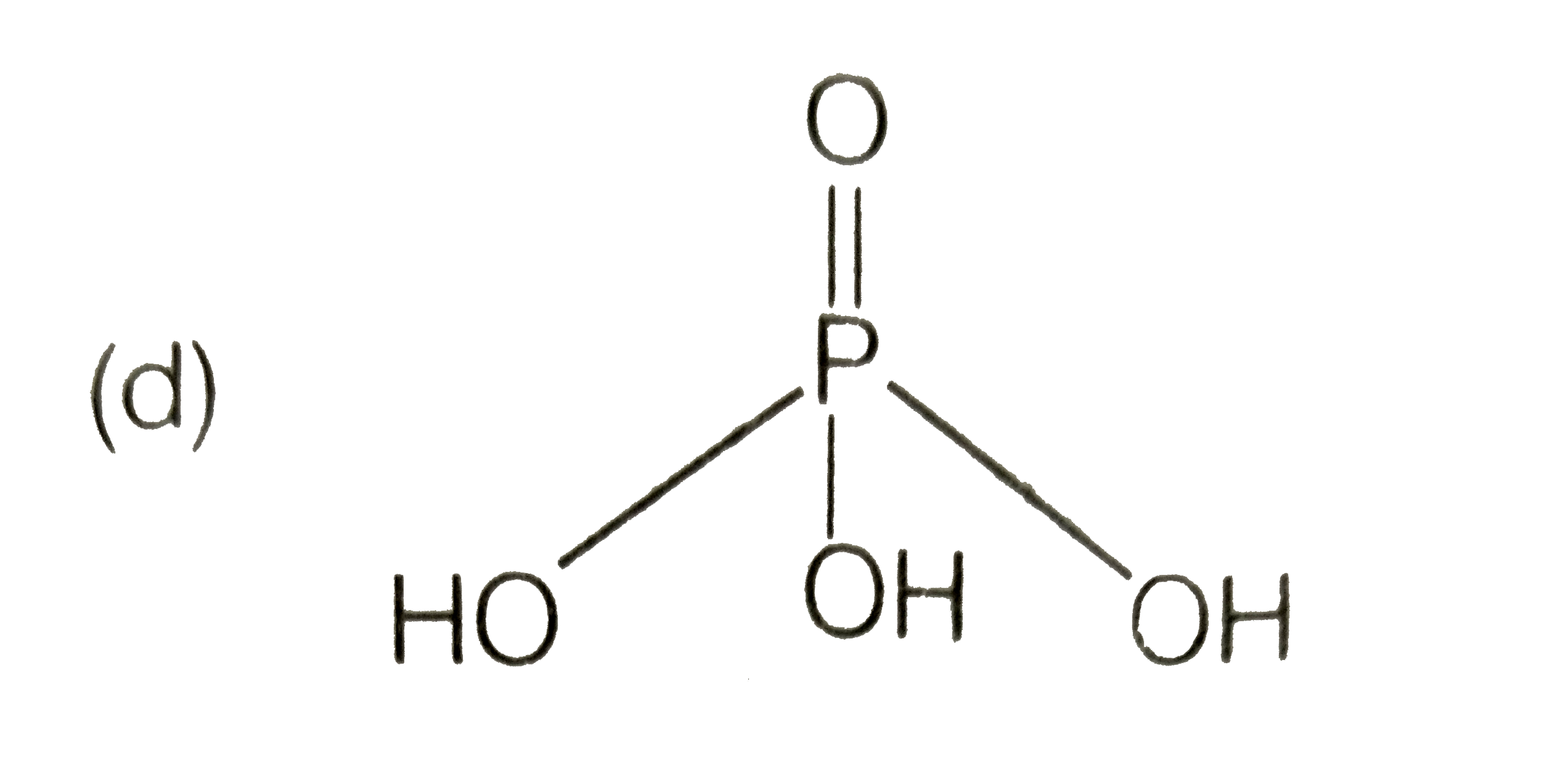
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- the correct structural formula of hypophosphorous acid is
Text Solution
|
- Hypophosphorous acid is
Text Solution
|
- The correct structural formula of hypophosphorous acid is
Text Solution
|
- The correct structural formula of butanoic acid is
Text Solution
|
- The structural formula of hypophosphorous acid is
Text Solution
|
- हाइपोफ़ॉस्फ़ोरिक अम्ल ............. बेसिक अम्ल है।
Text Solution
|
- The correct formula of salt formed by the neutraliation of hypophosph...
Text Solution
|
- Draw the structure of Hypophosphoric acid
Text Solution
|
- হাইপোফসফরাস এসিড ও এর প্রশমনে উৎপন্ন লবণটি হল-
Text Solution
|