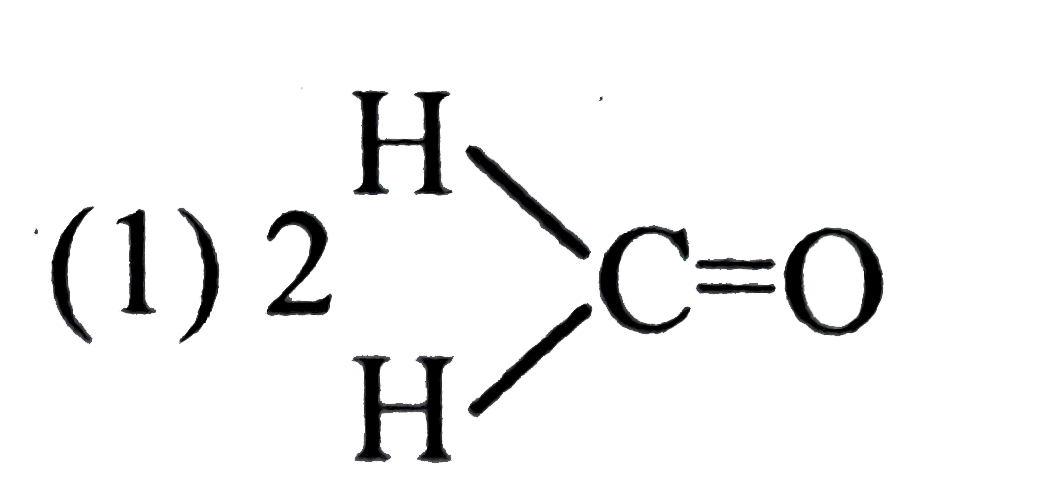
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CH(2)OHCH(2)OH on heating with periodic acid gives
Text Solution
|
- CH(2)OHCH(2)OH on heating with periodic acid gives
Text Solution
|
- CH(2)-OHCH(2)CH(2)CH(2)OH
Text Solution
|
- H(2)COH-CH(2)OH on heating with periodic acid gives
Text Solution
|
- CH(2)OHCH(2)CH(2)COCH(3)overset("Reagent")rarr CH(2)OHCH(2)CH(2)CH(2)C...
Text Solution
|
- CH(2)OH.CH(2)OH on heating with periodic acid gives
Text Solution
|
- H(2)COH-CH(2)OH on heating with periodic acid gives
Text Solution
|
- HO CH(2) CH(2)-OH on heating with periodic acid gives
Text Solution
|
- Identify the isomerism in each of the following paris CH(3)OCH(2)CH(3)...
Text Solution
|