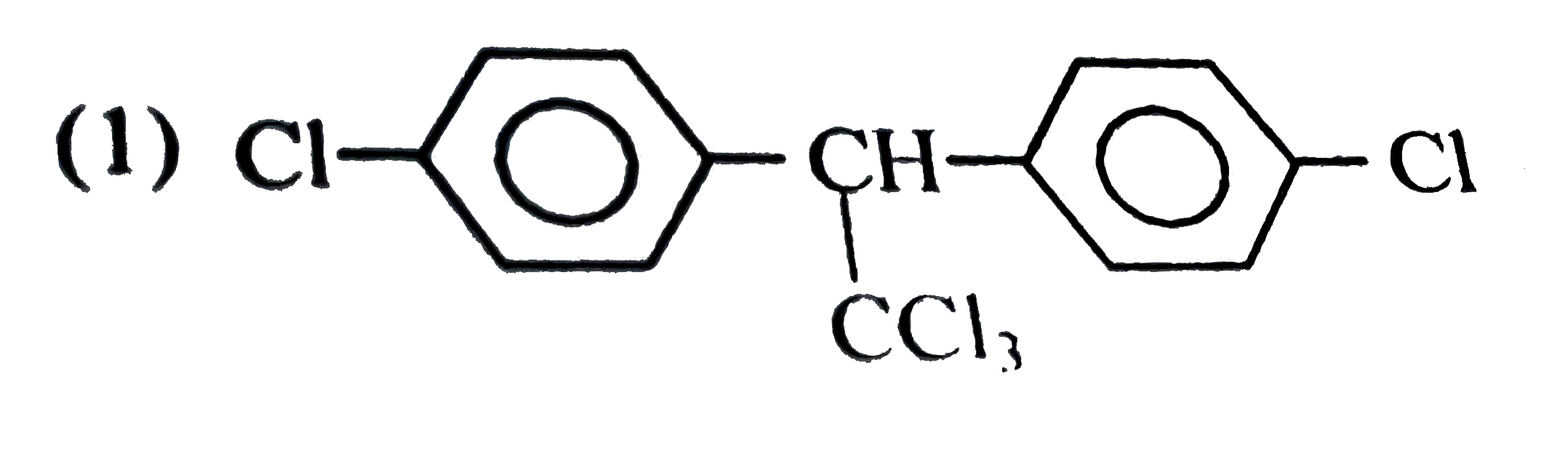
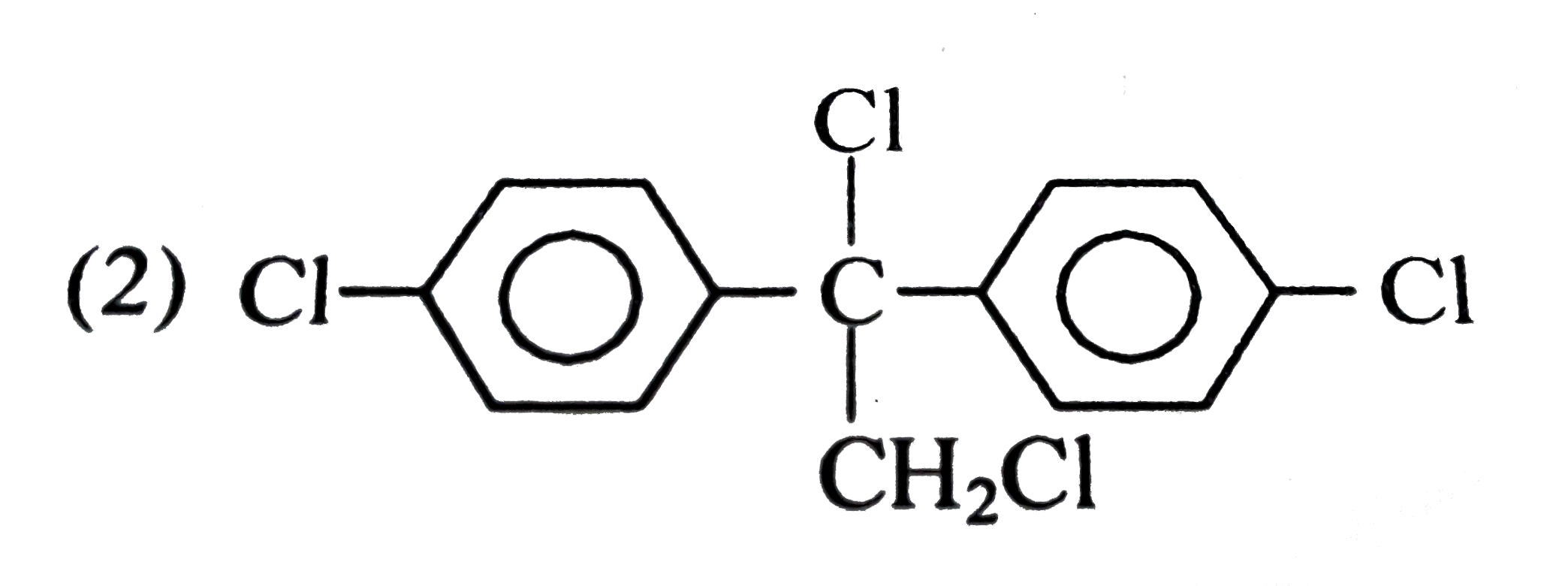
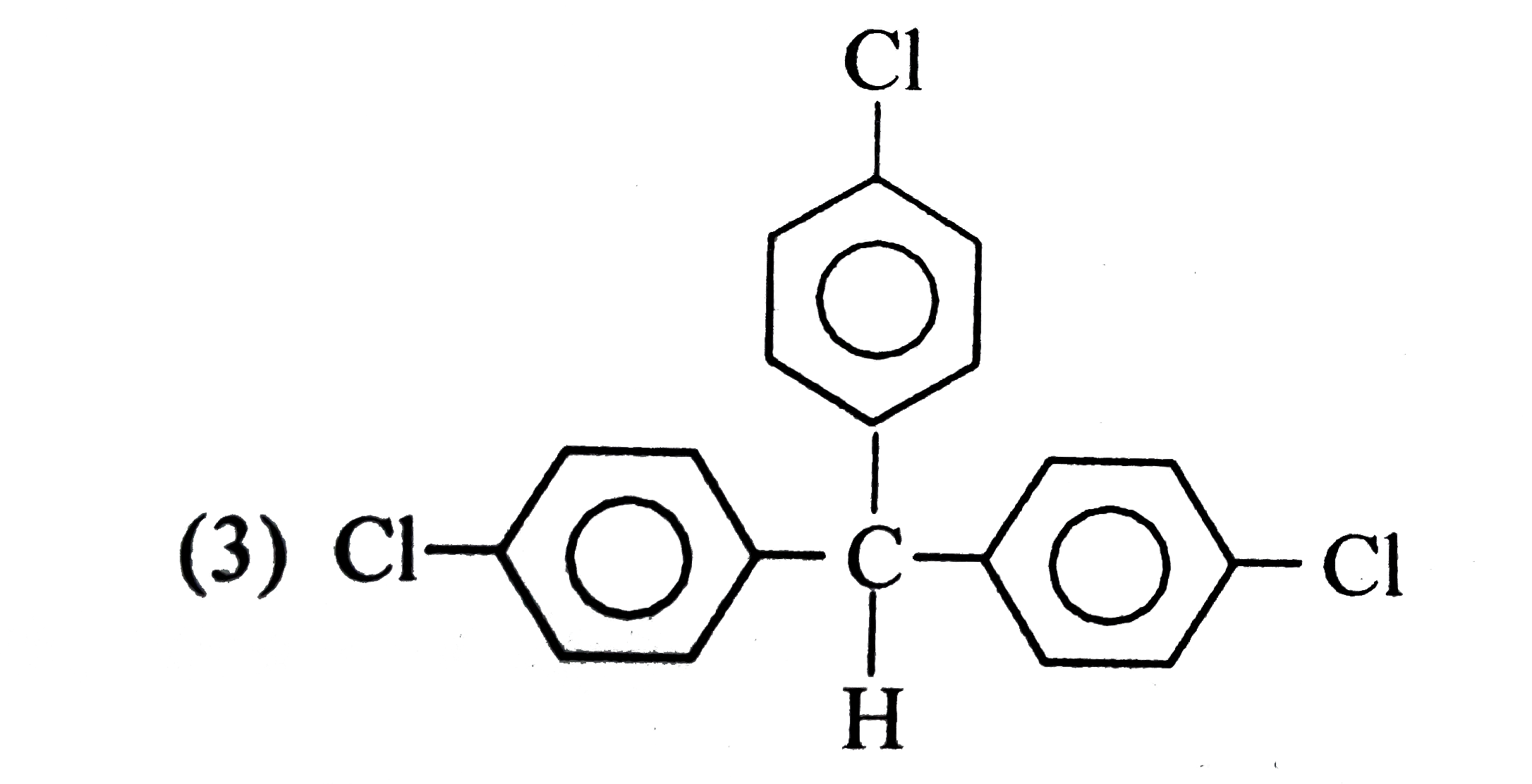
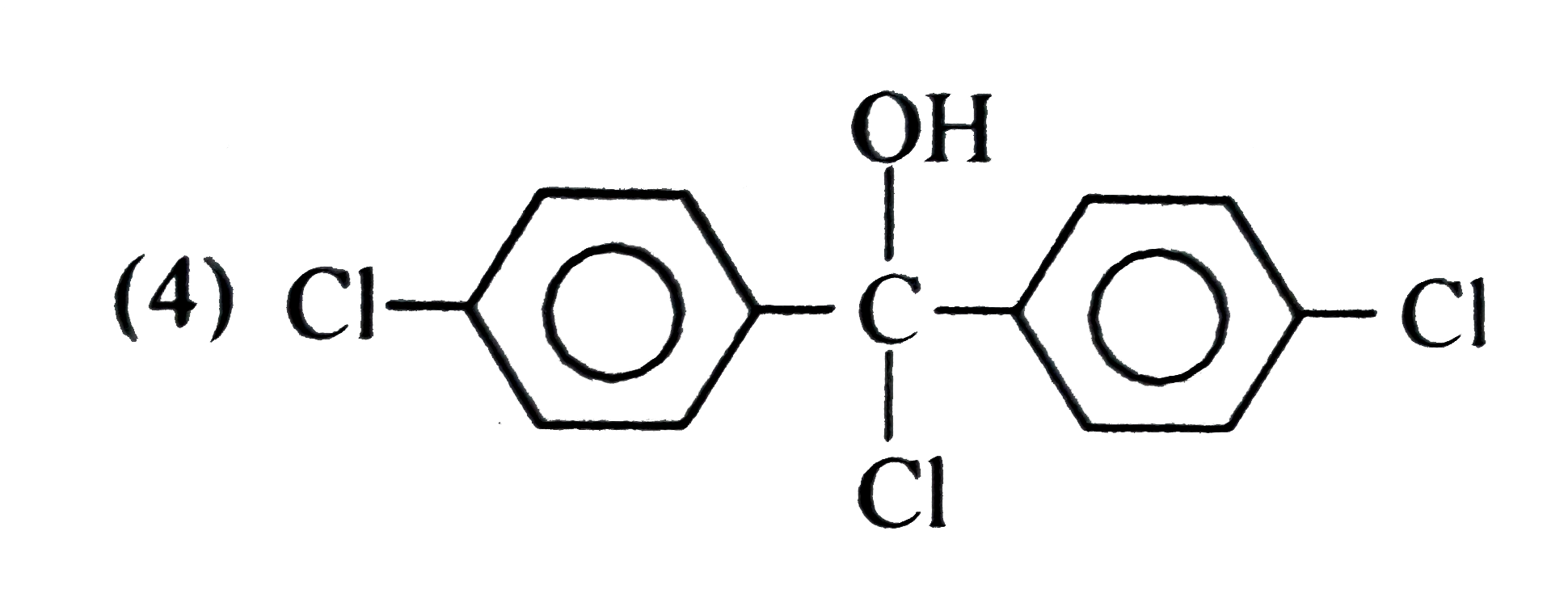
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Trichloroacetaldehyde, C Cl(3)CHO reacts with chlorobenzene in presenc...
Text Solution
|
- Trichlorroacetaldehyde, C CI(3)CHO. Reacts with chlorobenzene in prese...
Text Solution
|
- The compound formed on heating chlorobenzen with chloral in the prese...
Text Solution
|
- Chlorobenzene reacts with trichloroacetaldehyde in the presence of H2S...
Text Solution
|
- Trichloroacetaldehyde, C CI(3)CHO reacts with chlorobenzene in presenc...
Text Solution
|
- Trichloroacetaldehyde, C Cl(3)CHO reacts with chlorobenzene in presenc...
Text Solution
|
- In the presence of concentrated sulphuric acid acetic acid reacts wit...
Text Solution
|
- ट्राइक्लोरोएसीटैल्डिहाइड, C Cl(3)CHO, सल्फ्यूरिक अम्ल की उपस्थिति में ...
Text Solution
|
- To get DDT chlorobenzene has to react with the following compound in t...
Text Solution
|